Palladium-Catalyzed Synthesis of N-Aryl Pyrrolidines from γ-(N-Arylamino) Alkenes: Evidence for Chemoselective Alkene Insertion into PdN Bonds†
This research was supported by the University of Michigan. J.P.W. thanks The Dreyfus Foundation for a New Faculty Award and Research Corporation for an Innovation Award. J.E.N. thanks the University of Michigan for a Regents Fellowship. Additional unrestricted support was provided by Eli Lilly, 3M, and Amgen.
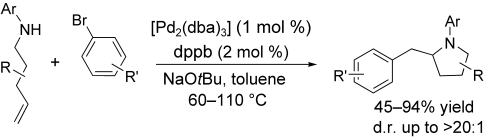
Graphical Abstract
The formation of a CC and a CN bond in a reaction between γ-(N-arylamino) alkenes and aryl bromides results in the stereoselective synthesis of substituted pyrrolidine derivatives (see scheme). Preliminary studies suggest these reactions proceed by intramolecular alkene insertion into the PdN bond of intermediate [Pd(Ar)(amido)] complexes. dba=dibenzylideneacetone, dppb=1,3-bis(diphenylphosphanyl)butane.