A Straightforward and Mild Synthesis of Functionalized 3-Alkynoates†
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, R01-GM066960), the Spanish Ministry of Education, Culture, and Sports (postdoctoral fellowship to A.S.), Merck Research Laboratories, and Novartis. Funding for the MIT Department of Chemistry Instrumentation Facility has been furnished in part by NSF CHE-9808061 and NSF DBI-9729592.
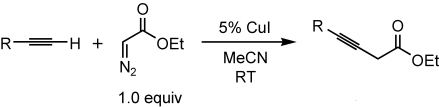
Graphical Abstract
Diazoacetates in coupling reactions: CuI serves as an effective catalyst for coupling terminal alkynes with diazo compounds to generate 3-alkynoates (see scheme). This method is efficient (1:1 ratio of reactants), mild (room temperature), and simple (no additional ligand), and a range of functional groups are tolerated (e.g., CC double bonds, heteroatoms, and hydroxy groups).