Rhodium(III)-Catalyzed Synthesis of Skipped Enynes via C(sp3)–H Alkynylation of Terminal Alkenes
Dr. Franco Della-Felice
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorMargherita Zanini
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorDr. Xiaoming Jie
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorEric Tan
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorCorresponding Author
Prof. Antonio M. Echavarren
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorDr. Franco Della-Felice
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorMargherita Zanini
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorDr. Xiaoming Jie
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorEric Tan
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorCorresponding Author
Prof. Antonio M. Echavarren
Institute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorAbstract
The RhIII-catalyzed allylic C−H alkynylation of non-activated terminal alkenes leads selectively to linear 1,4-enynes at room-temperature. The catalytic system tolerates a wide range of functional groups without competing functionalization at other positions. Similarly, the vinylic C−H alkynylation of α,β- and β,γ- unsaturated amides gives conjugated Z-1,3-enynes and E-enediynes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange202014877-sup-0001-misc_information.pdf10.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For representative reviews on directing group-assisted C−H functionalization, see:
- 1aZ. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu, Y. Zhang, Org. Chem. Front. 2015, 2, 1107–1295;
- 1bSambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes, M. Schnürch, Chem. Soc. Rev. 2018, 47, 6603–6743;
- 1cL. D. Caspers, B. J. Nachtsheim, Chem. Asian J. 2018, 13, 1231–1247;
- 1dO. K. Rasheed, B. Sun, ChemistrySelect 2018, 3, 5689–5708;
- 1eM. Kapoor, A. Singh, K. Sharma, M. Hua Hsu, Adv. Synth. Catal. 2020, 362, 4513–4542.
- 2For selected reviews on transient directing groups, see:
- 2aQ. Zhao, T. Poisson, X. Pannecoucke, T. Besset, Synthesis 2017, 49, 4808–4826;
- 2bP. Gandeepan, L. Ackermann, Chem 2018, 4, 199–222;
- 2cT. Bhattacharya, S. Pimparkar, D. Maiti, RSC Adv. 2018, 8, 19456–19464; For selected recent examples on remote C−H bond functionalization, see:
- 2dH. Shi, Y. Lu, J. Weng, K. L. Bay, X. Chen, K. Tanaka, P. Verma, K. N. Houk, J. Q. Yu, Nat. Chem. 2020, 12, 399–404;
- 2eR. R. Annapureddy, C. Jandl, T. Bach, J. Am. Chem. Soc. 2020, 142, 7374–7378.
- 3For reviews, see:
- 3aR. H. Crabtree, J. Chem. Soc. Dalton Trans. 2001, 2437–2450;
- 3bJ. A. Labinger, J. E. Bercaw, Nature 2002, 417, 507–514;
- 3cR. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer, O. Baudoin, Chem. Eur. J. 2010, 16, 2654–2672;
- 3dJ. Zhang, L. J. Kang, T. C. Parker, S. B. Blakey, C. K. Luscombe, S. R. Marder, Molecules 2018, 23, 922;
- 3eS. Yuan, Y. Li, J. Peng, Y. M. Questell-Santiago, K. Akkiraju, L. Giordano, D. J. Zheng, S. Bagi, Y. Román-Leshkov, Y. Shao-Horn, Adv. Energy Mater. 2020, 10, 2002154;
- 3fS. Shaaban, C. Davies, H. Waldmann, Eur. J. Org. Chem. 2020, 6512–6524.
- 4For reviews, see:
- 4aJ. Yamaguchi, A. D. Yamaguchi, K. Itami, Angew. Chem. Int. Ed. 2012, 51, 8960–9009; Angew. Chem. 2012, 124, 9092–9142;
- 4bE. J. E. Caro-Diaz, M. Urbano, D. J. Buzard, R. M. Jones, Bioorg. Med. Chem. Lett. 2016, 26, 5378–5383;
- 4cJ. Yamaguchi, K. Amaike, K. Itami in Transition Metal Heterocycle Synthesis via C−H Activation (Ed.: X.-F. Wu), Wiley-VCH, Weinheim, 2016, pp. 505–550;
- 4dD. Basu, S. Kumar, V. SaiSudhir, R. Bandichhor, J. Chem. Sci. 2018, 130, 71;
- 4eM. Moir, J. J. Danon, T. A. Reekie, M. Kassiou, Expert Opin. Drug Discovery 2019, 14, 1137–1149.
- 5For reviews, see:
- 5aK. Godula, D. Sames, Science 2006, 312, 67–72;
- 5bW. R. Gutekunst, P. S. Baran, Chem. Soc. Rev. 2011, 40, 1976–1991;
- 5cD. Y. K. Chen, S. W. Youn, Chem. Eur. J. 2012, 18, 9452–9474;
- 5dP. Tao, Y. Jia, Sci. China Chem. 2016, 59, 1109–1125;
- 5eK. Chen, X. Lei, Curr. Opin. Green Sustain. Chem. 2018, 11, 9–14;
- 5fS. K. Sinha, G. Zanoni, D. Maiti, Asian J. Org. Chem. 2018, 7, 1178–1192;
- 5gH. Zhai, Y. Li, F. Fang in Efficiency in Natural Product Total Synthesis (Eds.: P.-Q. Huang, Z.-J. Yao, R. P. Hsung), Wiley, Hoboken, 2018, pp. 261–272;
10.1002/9781118940228.ch5 Google Scholar
- 5hD. J. Abrams, P. A. Provencher, E. J. Sorensen, Chem. Soc. Rev. 2018, 47, 8925–8967.
- 6For reviews, see;
- 6aG. Song, F. Wang, X. Li, Chem. Soc. Rev. 2012, 41, 3651–3678;
- 6bG. Song, X. Li, Acc. Chem. Res. 2015, 48, 1007–1020;
- 6cS. Rej, N. Chatani, Angew. Chem. Int. Ed. 2019, 58, 8304–8329; Angew. Chem. 2019, 131, 8390–8416;
- 6dM. R. Sk, S. S. Bera, S. Basuli, A. Metya, M. S. Maji, Asian J. Org. Chem. 2020, 9, 1701–1717.
- 7For reviews, see:
- 7aT. G. Saint-Denis, R. Y. Zhu, G. Chen, Q. F. Wu, J. Q. Yu, Science 2018, 359, eaao4798;
- 7bJ. C. K. Chu, T. Rovis, Angew. Chem. Int. Ed. 2018, 57, 62–101; Angew. Chem. 2018, 130, 64–105.
- 8For an outlook on undirected C−H functionalization, see:
- 8aJ. F. Hartwig, M. A. Larsen, ACS Cent. Sci. 2016, 2, 281–292; for recent examples on undirected C−H functionalization, see:
- 8bA. Mondal, H. Chen, L. Flämig, P. Wedi, M. Van Gemmeren, J. Am. Chem. Soc. 2019, 141, 18662–18667;
- 8cR. Oeschger, B. Su, I. Yu, C. Ehinger, E. Romero, S. He, J. Hartwig, Science 2020, 368, 736–741.
- 9For representative reviews on undirected Pd-catalyzed π-allyl C−H bond functionalization of non-activated alkenes, see:
- 9aC. J. Engelin, P. Fristrup, Molecules 2011, 16, 951–969;
- 9bA. Breder, Synlett 2014, 25, 899–904;
- 9cF. Liron, J. Oble, M. M. Lorion, G. Poli, Eur. J. Org. Chem. 2014, 5863–5883;
- 9dR. A. Fernandes, J. L. Nallasivam, Org. Biomol. Chem. 2019, 17, 8647–8672.
- 10For literature related to isolated CpXM (M=Rh, Ir) complexes, see:
- 10aR. A. Periana, R. G. Bergman, J. Am. Chem. Soc. 1984, 106, 7272–7273;
- 10bJ. B. Wakefield, J. M. Stryker, J. Am. Chem. Soc. 1991, 113, 7057–7059;
- 10cY. F. Han, G. X. Jin, Chem. Soc. Rev. 2014, 43, 2799–2823;
- 10dY. Shibata, E. Kudo, H. Sugiyama, H. Uekusa, K. Tanaka, Organometallics 2016, 35, 1547–1552;
- 10eA. Lerchen, T. Knecht, M. Koy, J. B. Ernst, K. Bergander, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2018, 57, 15248–15252; Angew. Chem. 2018, 130, 15468–15472;
- 10fR. J. Harris, J. Park, T. A. F. Nelson, N. Iqbal, D. C. Salgueiro, J. Bacsa, C. E. Macbeth, M. H. Baik, S. B. Blakey, J. Am. Chem. Soc. 2020, 142, 5842–5851.
- 11T. Cochet, V. Bellosta, D. Roche, J. Y. Ortholand, A. Greiner, J. Cossy, Chem. Commun. 2012, 48, 10745–10747.
- 12
- 12aA. Archambeau, T. Rovis, Angew. Chem. Int. Ed. 2015, 54, 13337–13340; Angew. Chem. 2015, 127, 13535–13538;
- 12bQ. Cui, W. Liao, Z. Y. Tian, Q. Li, Z. X. Yu, Org. Lett. 2019, 21, 7692–7696;
- 12cT. A. F. Nelson, M. R. Hollerbach, S. B. Blakey, Dalton Trans. 2020, 49, 13928–13935.
- 13For the preparation of 1,4-enynes from non-activated alkenes, see:
- 13aX. Le Zhou, L. Ren, P. S. Wang, J. Org. Chem. 2017, 82, 9794–9800;
- 13bA. A. Almasalma, E. Mejía, Chem. Eur. J. 2018, 24, 12269–12273.
- 14For selected systematic studies on transformations of 1,4-enynes, see:
- 14aA. Buzas, F. Gagosz, J. Am. Chem. Soc. 2006, 128, 12614–12615;
- 14bS. Gao, H. Liu, C. Yang, Z. Fu, H. Yao, A. Lin, Org. Lett. 2017, 19, 4710–4713;
- 14cX. F. Wei, X. W. Xie, Y. Shimizu, M. Kanai, J. Am. Chem. Soc. 2017, 139, 4647–4650;
- 14dX. Fang, Q. Li, R. Shi, H. Yao, A. Lin, Org. Lett. 2018, 20, 6084–6088.
- 15For systematic studies on 1,4-enyne formation by cross-coupling, see:
- 15aA. Commercon, J. Normant, J. Villieras, J. Organomet. Chem. 1975, 93, 415–421;
- 15bT. P. Heffron, J. D. Trenkle, T. F. Jamison, Tetrahedron 2003, 59, 8913–8917;
- 15cM. Shirakura, M. Suginome, Angew. Chem. Int. Ed. 2010, 49, 3827–3829; Angew. Chem. 2010, 122, 3915–3917;
- 15dR. Agata, S. Lu, H. Matsuda, K. Isozaki, M. Nakamura, Org. Biomol. Chem. 2020, 18, 3022–3026.
- 16For recent systematic studies on 1,4-enyne formation by allylic alkynylation, see:
- 16aA. Harada, Y. Makida, T. Sato, H. Ohmiya, M. Sawamura, J. Am. Chem. Soc. 2014, 136, 13932–13939;
- 16bQ. Yang, Y. Zhou, J. Chen, X. He, J. Xu, F. Y. Kwong, B. Fan, Eur. J. Org. Chem. 2015, 5330–5333;
- 16cT. Nakane, Y. Tanioka, N. Tsukada, Organometallics 2015, 34, 1191–1196;
- 16dP. Xie, Z. Sun, S. Li, L. Zhang, X. Cai, W. Fu, X. Yang, Y. Liu, X. Wo, T. P. Loh, Org. Lett. 2020, 22, 1599–1604.
- 17For selected systematic studies on 1,4-enyne formation by allylic alkylation, see
- 17aB. M. Trost, S. Hildbrand, K. Dogra, J. Am. Chem. Soc. 1999, 121, 10416–10417;
- 17bR. Takeuchi, K. Tanabe, Angew. Chem. Int. Ed. 2000, 39, 1975–1978;
10.1002/1521-3773(20000602)39:11<1975::AID-ANIE1975>3.0.CO;2-I CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 2051–2054;
- 17cM. A. Kacprzynski, A. H. Hoveyda, J. Am. Chem. Soc. 2004, 126, 10676–10681;
- 17dN. F. Langille, T. F. Jamison, Org. Lett. 2006, 8, 3761–3764;
- 17eH. Li, A. Alexakis, Angew. Chem. Int. Ed. 2012, 51, 1055–1058; Angew. Chem. 2012, 124, 1079–1082;
- 17fY. Makida, Y. Takayama, H. Ohmiya, M. Sawamura, Angew. Chem. Int. Ed. 2013, 52, 5350–5354; Angew. Chem. 2013, 125, 5458–5462.
- 18For systematic studies on 1,4-enyne formation nucleophilic substitution, see:
- 18aS. Peng, L. Wang, J. Wang, Org. Biomol. Chem. 2012, 10, 225–228;
- 18bG. Huang, X. Wang, Y. Pan, H. Wang, G. Yao, Y. Zhang, J. Org. Chem. 2013, 78, 2742–2745.
- 19
- 19aE. Tan, A. I. Konovalov, G. A. Fernandez, R. Dorel, A. M. Echavarren, Org. Lett. 2017, 19, 5561–5564;
- 19bE. Tan, O. Quinonero, M. E. de Orbe, A. M. Echavarren, ACS Catal. 2018, 8, 2166–2172;
- 19cE. Tan, M. Zanini, A. M. Echavarren, Angew. Chem. Int. Ed. 2020, 59, 10470–10473; Angew. Chem. 2020, 132, 10556–10559.
- 20For the selective formation of 1,1-bis alkylation compounds, see: Y. Li, H. Wei, D. Wu, Z. Li, W. Wang, G. Yin, ACS Catal. 2020, 10, 4888–4894.
- 21To the best of our knowledge, linear selectivity in Cp*RhIII-catalyzed allyl-aryl coupling is only favored in systems in which the final alkene is conjugated to an aryl ring:
- 21aref. [10e];
- 21bT. Knecht, T. Pinkert, T. Dalton, A. Lerchen, F. Glorius, ACS Catal. 2019, 9, 1253–1257.
- 22
- 22aP. M. Boyer, C. R. Roy, J. M. Bielski, J. S. Merola, Inorg. Chim. Acta 1996, 245, 7–15;
- 22bG. A. M. Jardim, E. N. Da Silva, J. F. Bower, Chem. Sci. 2016, 7, 3780–3784.
- 23See the Supporting Information for additional details.
- 24For the role of acetate in the cyclometallation with [Cp*RhCl2]2, [Cp*IrCl2]2, and [RuCl2(p-cymene)]2, see: D. L. Davies, O. Al-Duaij, J. Fawcett, M. Giardiello, S. T. Hilton, D. R. Russell, Dalton Trans. 2003, 4132–4138.
- 25See the Supporting Information for all the computational details and the complete study.
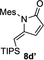
- 26Substrate 5 d also delivered 8 d′ in 7 % yield under these conditions.[23]
- 27For directed ortho-alkynylation of anylines, see:
- 27aR. Amemiya, A. Fujii, M. Yamaguchi, Tetrahedron Lett. 2004, 45, 4333–4335;
- 27bM. Tobisu, Y. Ano, N. Chatani, Org. Lett. 2009, 11, 3250–3252;
- 27cF. Xie, Z. Qi, S. Yu, X. Li, J. Am. Chem. Soc. 2014, 136, 4780–4787;
- 27dZ. Ruan, S. Lackner, L. Ackermann, ACS Catal. 2016, 6, 4690–4693.
- 28For the preparation of related conjugated allenes, see ref. [20].
- 29K. Yoshida, P. A. Grieco, Chem. Lett. 1985, 14, 155–158.
- 30
- 30aY. Hoshino, Y. Shibata, K. Tanaka, Angew. Chem. Int. Ed. 2012, 51, 9407–9411; Angew. Chem. 2012, 124, 9541–9545;
- 30bC. Feng, D. Feng, Y. Luo, T. P. Loh, Org. Lett. 2014, 16, 5956–5959.
- 31Since acceptance of this manuscript, a related study was published: S. Mondal, T. Pinkert, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2021, https://doi.org/10.1002/anie.202015249; Angew. Chem. 2021, https://doi.org/10.1002/ange.202015249.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.




