Gold-Catalyzed [3+2]-Annulations of α-Aryl Diazoketones with the Tetrasubstituted Alkenes of Cyclopentadienes: High Stereoselectivity and Enantioselectivity
Ching-Nung Chen
Frontier Research Center for Matter Science and Technology and Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, ROC
Search for more papers by this authorWei-Min Cheng
Frontier Research Center for Matter Science and Technology and Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, ROC
Search for more papers by this authorJian-Kai Wang
Frontier Research Center for Matter Science and Technology and Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, ROC
Search for more papers by this authorTzu-Hsuan Chao
Department of Chemistry, National Cheng Kung University, East District, Tainan City, Taiwan, ROC
Search for more papers by this authorCorresponding Author
Mu-Jeng Cheng
Department of Chemistry, National Cheng Kung University, East District, Tainan City, Taiwan, ROC
Search for more papers by this authorCorresponding Author
Rai-Shung Liu
Frontier Research Center for Matter Science and Technology and Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, ROC
Search for more papers by this authorChing-Nung Chen
Frontier Research Center for Matter Science and Technology and Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, ROC
Search for more papers by this authorWei-Min Cheng
Frontier Research Center for Matter Science and Technology and Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, ROC
Search for more papers by this authorJian-Kai Wang
Frontier Research Center for Matter Science and Technology and Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, ROC
Search for more papers by this authorTzu-Hsuan Chao
Department of Chemistry, National Cheng Kung University, East District, Tainan City, Taiwan, ROC
Search for more papers by this authorCorresponding Author
Mu-Jeng Cheng
Department of Chemistry, National Cheng Kung University, East District, Tainan City, Taiwan, ROC
Search for more papers by this authorCorresponding Author
Rai-Shung Liu
Frontier Research Center for Matter Science and Technology and Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, ROC
Search for more papers by this authorAbstract
This work reports gold-catalyzed [3+2]-annulations of α-diazo ketones with highly substituted cyclopentadienes, affording bicyclic 2,3-dihydrofurans with high regio- and stereoselectivity. The reactions highlights the first success of tetrasubstituted alkenes to undergo [3+2]-annulations with α-diazo carbonyls. The enantioselective annulations are also achieved with high enantioselectivity using chiral forms of gold and phosphoric acid. Our mechanistic analysis supports that cyclopentadienes serve as nucleophiles to attack gold carbenes at the more substituted alkenes, yielding gold enolates that complex with chiral phosphoric acid to enhance the enantioselectivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange202012611-sup-0001-misc_information.pdf14.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews for catalytic cyclopropanations, see:
- 1aH. Lebel, J. F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977–1050;
- 1bH. Pellissier, Tetrahedron 2008, 64, 7041–7095;
- 1cD. Qiana, J. Zhang, Chem. Soc. Rev. 2015, 44, 677–698;
- 1dW. Wu, Z. Lin, H. Jiang, Org. Biomol. Chem. 2018, 16, 7315–7329;
- 1eM. P. Doyle, D. C. Forbes, Chem. Rev. 1998, 98, 911–935.
- 2For applications to synthesis of bioactive molecules, see selected reviews:
- 2aP. Tang, Y. Qin, Synthesis 2012, 19, 2969–2984;
- 2bC. Ebner, E. M. Carreira, Chem. Rev. 2017, 117, 11651–11679;
- 2cT. F. Schneider, J. Kaschel, D. B. Werz, Angew. Chem. Int. Ed. 2014, 53, 5504–5523; Angew. Chem. 2014, 126, 5608–5628.
- 3For catalytic asymmetric cyclopropanation, see recent examples:
- 3aH. M. L. Davies, M. G. Coleman, D. L. Ventura, Org. Lett. 2007, 9, 4971–4974;
- 3bJ. F. Briones, H. M. L. Davies, J. Am. Chem. Soc. 2012, 134, 11916–11919;
- 3cZ.-Y. Cao, X. Wang, C. Tan, X. L. Zhao, J. Zhou, K. Ding, J. Am. Chem. Soc. 2013, 135, 8197–8200;
- 3dH. Xu, Y. P. Li, Y. Cai, G. P. Wang, S. F. Zhu, Q. L. Zhou, J. Am. Chem. Soc. 2017, 139, 7697–7700;
- 3eM. Garbo, C. Besnard, L. Guénée, C. Mazet, ACS Catal. 2020, 10, 9604–9611;
- 3fB. Wei, J. C. Sharland, P. Lin, S. M. W. Hill, F. A. Fullilove, S. McKinnon, D. G. Blackmond, H. M. L. Davies, ACS Catal. 2020, 10, 1161–1170.
- 4
- 4aC. H. Lin, D. Pursley, J. E. M. N. Klein, J. Teske, J. A. Allen, F. Rami, A. Köhn, B. Plietker, Chem. Sci. 2015, 6, 7034–7043;
- 4bJ. Zhang, Y. Tang, W. Wei, Y. Wu, Y. Li, J. Zhang, Y. Zheng, S. Xu, Org. Lett. 2017, 19, 3043–3046;
- 4cM. L. Piotrowski, M. A. Kerr, Org. Lett. 2018, 20, 7624–7627.
- 5A. Ortega, R. Manzano, U. Uria, L. Carrillo, E. Reyes, T. Tejero, P. Merino, J. L. Vicario, Angew. Chem. Int. Ed. 2018, 57, 8225–8229; Angew. Chem. 2018, 130, 8357–8361.
- 6
- 6aH. M. L. Davies, G. Ahmed, R. L. Calvo, M. R. Churchill, D. G. Churchill, J. Org. Chem. 1998, 63, 2641–2645;
- 6bL. Xiaa, Y. R. Lee, Adv. Synth. Catal. 2013, 355, 2361–2374.
- 7Only one report was noted for the olefination of α-diazo carbonyl with tetrasubstituted enol ethers, but giving niether cyclopropanation nor [3+2]-annulation products. See: F. M. Liao, Z. Y. Cao, J. S. Yu, J. Zhou, Angew. Chem. Int. Ed. 2017, 56, 2459–2463; Angew. Chem. 2017, 129, 2499–2503.
- 8
- 8aJ. Sun, D. Shi, M. Ma, S. Li, S. Wang, L. Han, Y. Yang, X. Fan, J. Shi, L. He, J. Nat. Prod. 2005, 68, 915–919;
- 8bM. Umehara, A. Hanada, S. Yoshida, K. Akiyama, T. Arite, N. Takeda-Kamiya, H. Magome, Y. Kamiya, K. Shirasu, K. Yoneyama, J. Kyozuka, S. Yamaguchi, Nature 2008, 455, 195–200;
- 8cJ. A. Malona, K. Cariou, W. T. Spencer III, A. J. Frontier, J. Org. Chem. 2012, 77, 1891–1908;
- 8dL. Pan, L. B. S. Kardono, S. Riswan, H. Chai, E. J. Carcache de Blanco, C. M. Pannell, D. D. Soejarto, T. G. McCloud, D. J. Newman, A. D. Kinghorn, J. Nat. Prod. 2010, 73, 1873–1878.
- 9
- 9aC. N. Chen, R. S. Liu, Angew. Chem. Int. Ed. 2019, 58, 9831–9835; Angew. Chem. 2019, 131, 9936–9940;
- 9bP. D. Jadhav, J. X. Chen, R. S. Liu, ACS Catal. 2020, 10, 5840–5845;
- 9cB. D. Mokar, P. D. Jadhav, Y. B. Pandit, R. S. Liu, Chem. Sci. 2018, 9, 4488–4492;
- 9dH. Funami, H. Kusama, N. Iwasawa, Angew. Chem. Int. Ed. 2007, 46, 909–911; Angew. Chem. 2007, 119, 927–929;
- 9eJ. H. Lee, F. D. Toste, Angew. Chem. Int. Ed. 2007, 46, 912–914; Angew. Chem. 2007, 119, 930–932.
- 10Deposition Numbers 2026964 and 2026956 (for 5e and (+)-6q) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 11For gold-catalyzed asymmetric annulations of 1,3-dienes; see:
- 11aP. Mauleón, R. M. Zeldin, A. Z. González, F. D. Toste, J. Am. Chem. Soc. 2009, 131, 6348–6349;
- 11bI. Alonso, B. Trillo, F. López, S. Montserrat, G. Ujaque, L. Castedo, A. Lledós, J. L. Mascareñas, J. Am. Chem. Soc. 2009, 131, 13020–13030;
- 11cA. Z. González, F. D. Toste, Org. Lett. 2010, 12, 200–203;
- 11dF. López, J. L. Mascareñas, Beilstein J. Org. Chem. 2013, 9, 2250–2264;
- 11eH. Faustino, F. López, L. Castedo, J. L. Mascareñas, Chem. Sci. 2011, 2, 633–637;
- 11fI. Alonso, H. Faustino, F. López, J. L. Mascareñas, Angew. Chem. Int. Ed. 2011, 50, 11496–11500; Angew. Chem. 2011, 123, 11698–11702;
- 11gJ. Francos, F. G. Carmona, H. Faustino, J. I. Sigüenza, E. Díez, I. Alonso, R. Fernández, J. M. Lassaletta, F. López, J. L. Mascareñas, J. Am. Chem. Soc. 2012, 134, 14322–14325;
- 11hJ. F. Brazeau, S. Zhang, I. Colomer, B. K. Corkey, F. D. Toste, J. Am. Chem. Soc. 2012, 134, 2742–2749.
- 12For gold asymmetric catalysis, see recent reviews:
- 12aW. Zi, F. D. Toste, Chem. Soc. Rev. 2016, 45, 4567–4589;
- 12bP. Y. Toullec, A. Pradal, V. Michelet in Gold Catalysis An Homogeneous Approch, Vol. 13 (Ed.: F. D. Toste, V. Michelet), Imperial College Press, London, 2014, pp. 445–500;
- 12cY. Li, W. Li, J. Zhang, Chem. Eur. J. 2017, 23, 467–512;
- 12dY.-M. Wang, A. D. Lackner, F. D. Toste, Acc. Chem. Res. 2014, 47, 889–901;
- 12eG. L. Hamilton, E. J. Kang, M. Mba, F. D. Toste, Science 2007, 317, 496–499.
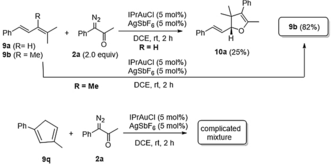
- 13Acyclic trisubstituted diene 9 a yielded the annulation products 10 a in 25 % yield, but the annulation was inapplicable to tetrasubstituted acyclic diene 9 b with a 82 % recovery. We also prepared 1,3-disubstituted cyclopentadiene 9 q that reacted with α-diazo ketone 2 a to give several minor products. Additional efforts to optimize this reaction are underway.
- 14Additional data to manifest the effects of solvents, phosphoric acids, and silver salts on asymmetric [3+2]-annulations are provided in the Supporting Information, Tables S2, S3 and Eqs. (s1),(s2).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.




