Copper-Catalyzed Site-Selective Intramolecular Amidation of Unactivated C(sp3)H Bonds†
Correction(s) for this article
-
Berichtigung: Copper-Catalyzed Site-Selective Intramolecular Amidation of Unactivated C(sp3)H Bonds
- Volume 127Issue 9Angewandte Chemie
- pages: 2615-2615
- First Published online: February 17, 2015
Dr. Xuesong Wu
Department of Chemistry and Chemical Biology, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202 (USA)
Search for more papers by this authorYan Zhao
Department of Chemistry and Chemical Biology, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202 (USA)
Search for more papers by this authorDr. Guangwu Zhang
Department of Chemistry and Chemical Biology, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Haibo Ge
Department of Chemistry and Chemical Biology, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202 (USA)
Institute of Chemistry and BioMedical Sciences and School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (P.R. China)
Department of Chemistry and Chemical Biology, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202 (USA)Search for more papers by this authorDr. Xuesong Wu
Department of Chemistry and Chemical Biology, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202 (USA)
Search for more papers by this authorYan Zhao
Department of Chemistry and Chemical Biology, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202 (USA)
Search for more papers by this authorDr. Guangwu Zhang
Department of Chemistry and Chemical Biology, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Haibo Ge
Department of Chemistry and Chemical Biology, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202 (USA)
Institute of Chemistry and BioMedical Sciences and School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093 (P.R. China)
Department of Chemistry and Chemical Biology, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202 (USA)Search for more papers by this authorWe gratefully acknowledge Indiana University Purdue University Indianapolis for financial support. The Bruker 500 MHz NMR was purchased using funds from an NSF-MRI award (CHE-0619254).
Abstract
The intramolecular dehydrogenative amidation of aliphatic amides, directed by a bidentate ligand, was developed using a copper-catalyzed sp3 CH bond functionalization process. The reaction favors predominantly the CH bonds of β-methyl groups over the unactivated methylene CH bonds. Moreover, a preference for activating sp3 CH bonds of β-methyl groups, via a five-membered ring intermediate, over the aromatic sp2 CH bonds was also observed in the cyclometalation step. Additionally, sp3 CH bonds of unactivated secondary sp3 CH bonds could be functionalized by favoring the ring carbon atoms over the linear carbon atoms.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201311263_sm_miscellaneous_information.pdf3.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected recent reviews, see:
- 1aO. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074;
- 1bX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196; Angew. Chem. Int. Ed. 2009, 48, 5094;
- 1cM. Wasa, K. M. Engle, J.-Q. Yu, Isr. J. Chem. 2010, 50, 605;
- 1dT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 1eR. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer, O. Baudoin, Chem. Eur. J. 2010, 16, 2654;
- 1fL. Ackermann, Chem. Commun. 2010, 46, 4866;
- 1gH. Li, B.-J. Li, Z.-J. Shi, Catal. Sci. Technol. 2011, 1, 191;
- 1hN. Yoshikai, Synlett 2011, 1047;
- 1iH. M. L. Davies, J. Du Bois, J.-Q. Yu, Chem. Soc. Rev. 2011, 40, 1855;
- 1jW. R. Gutekunst, P. S. Baran, Chem. Soc. Rev. 2011, 40, 1976;
- 1kJ. F. Hartwig, Chem. Soc. Rev. 2011, 40, 1992;
- 1lT. Newhouse, P. S. Baran, Angew. Chem. 2011, 123, 3422;
10.1002/ange.201006368 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3362;
- 1mO. Baudoin, Chem. Soc. Rev. 2011, 40, 4902;
- 1nM. C. White, Science 2012, 335, 807;
- 1oD. A. Colby, A. S. Tsai, R. G. Bergman, J. A. Ellman, Acc. Chem. Res. 2012, 45, 814.
- 2G. Rouquet, N. Chatani, Angew. Chem. 2013, 125, 11942;
10.1002/ange.201301451 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 11726.
- 3B. D. Dangel, K. Godula, S. W. Youn, B. Sezen, D. Sames, J. Am. Chem. Soc. 2002, 124, 11856.
- 4
- 4aV. Zaitsev, D. Shabashov, O. Daugulis, J. Am. Chem. Soc. 2005, 127, 13154;
- 4bB. V. S. Reddy, L. R. Reddy, E. J. Corey, Org. Lett. 2006, 8, 3391;
- 4cR. Giri, N. Maugel, B. M. Foxman, J.-Q. Yu, Organometallics 2008, 27, 1667;
- 4dD. Shabashov, O. Daugulis, J. Am. Chem. Soc. 2010, 132, 3965;
- 4eG. He, G. Chen, Angew. Chem. 2011, 123, 5298; Angew. Chem. Int. Ed. 2011, 50, 5192;
- 4fY. Ano, M. Tobisu, N. Chatani, J. Am. Chem. Soc. 2011, 133, 12984;
- 4gG. He, Y.-S. Zhao, S.-Y. Zhang, C.-X. Lu, G. Chen, J. Am. Chem. Soc. 2012, 134, 3;
- 4hE. T. Nadres, O. Daugulis, J. Am. Chem. Soc. 2012, 134, 7;
- 4iY. Ano, M. Tobisu, N. Chatani, Org. Lett. 2012, 14, 354;
- 4jR. K. Rit, M. R. Yadav, A. K. Sahoo, Org. Lett. 2012, 14, 3724;
- 4kL. D. Tran, O. Daugulis, Angew. Chem. 2012, 124, 5278; Angew. Chem. Int. Ed. 2012, 51, 5188;
- 4lN. Rodríguez, J. A. Romero-Revilla, M. Á. Fernández-Ibáñez, J. C. Carretero, Chem. Sci. 2013, 4, 175;
- 4mS.-Y. Zhang, G. He, W. A. Nack, Y. Zhao, Q. Li, G. Chen, J. Am. Chem. Soc. 2013, 135, 2124;
- 4nK. Chen, F. Hu, S.-Q. Zhang, B.-F. Shi, Chem. Sci. 2013, 4, 3906;
- 4oG. He, S.-Y. Zhang, W. A. Nack, Q. Li, G. Chen, Angew. Chem. 2013, 125, 11330; Angew. Chem. Int. Ed. 2013, 52, 11124;
- 4pQ. Zhang, K. Chen, W.-H. Rao, Y.-J. Zhang, F.-J. Chen, B.-F. Shi, Angew. Chem. 2013, 125, 13833; Angew. Chem. Int. Ed. 2013, 52, 13588;
- 4qW.-W. Sun, P. Cao, R.-Q. Mei, Y. Li, Y.-L. Ma, B. Wu, Org. Lett. 2014, 16, 480.
- 5
- 5aN. Hasegawa, V. Charra, S. Inoue, Y. Fukumoto, N. Chatani, J. Am. Chem. Soc. 2011, 133, 8070;
- 5bN. Hasegawa, K. Shibata, V. Charra, S. Inoue, Y. Fukumoto, N. Chatani, Tetrahedron 2013, 69, 4466.
- 6R. Shang, L. Ilies, A. Matsumoto, E. Nakamura, J. Am. Chem. Soc. 2013, 135, 6030.
- 7X.-S. Wu, Y. Zhao, H.-B. Ge, J. Am. Chem. Soc. 2014, 136, 1789.
- 8
- 8aX. Chen, X.-S. Hao, C. E. Goodhue, J.-Q. Yu, J. Am. Chem. Soc. 2006, 128, 6790;
- 8bM. Wasa, J.-Q. Yu, J. Am. Chem. Soc. 2008, 130, 14058;
- 8cL. D. Tran, I. Popov, O. Daugulis, J. Am. Chem. Soc. 2012, 134, 18237;
- 8dT. Truong, K. Klimovica, O. Daugulis, J. Am. Chem. Soc. 2013, 135, 9342;
- 8eJ. Roane, O. Daugulis, Org. Lett. 2013, 15, 5842;
- 8fL. D. Tran, J. Roane, O. Daugulis, Angew. Chem. 2013, 125, 6159; Angew. Chem. Int. Ed. 2013, 52, 6043.
- 9For selected recent reviews, see:
- 9aG. S. Singh, M. D′hooghe, N. De Kimpe, Tetrahedron 2011, 67, 1989;
- 9bA. MacGowan, Curr. Opin. Pharmacol. 2011, 11, 470;
- 9cP. Galletti, D. Giacomini, Curr. Med. Chem. 2011, 18, 4265;
- 9dA. Kamath, I. Ojima, Tetrahedron 2012, 68, 10640;
- 9eS. G. Kurz, R. A. Bonomo, Expert Rev. Anti-Infect. Ther. 2012, 10, 999;
- 9fI. Balderas-Renteria, P. Gonzalez-Barranco, A. Garcia, B. K. Banik, G. Rivera, Curr. Med. Chem. 2012, 19, 4377;
- 9gT. Orbegozo, F. Burel, P. Jubault, X. Pannecoucke, Tetrahedron 2013, 69, 4015;
- 9hK. Tahlan, S. E. Jensen, J. Antibiot. 2013, 66, 401;
- 9iD. A. Leonard, R. A. Bonomo, R. A. Powers, Acc. Chem. Res. 2013, 46, 2407;
- 9jJ. Chen, X.-H. Shang, F. Hu, X.-Z. Lao, X.-D. Gao, H. Zheng, W.-B. Yao, Mini-Rev. Med. Chem. 2013, 13, 1846.
- 10For selected recent reviews, see:
- 10aC.-J. Li, Acc. Chem. Res. 2009, 42, 335;
- 10bW.-J. Yoo, C.-J. Li, Top. Curr. Chem. 2010, 292, 281;
- 10cC. J. Scheuermann, Chem. Asian J. 2010, 5, 436;
- 10dC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215;
- 10eC. Liu, H. Zhang, W. Shi, A.-W. Lei, Chem. Rev. 2011, 111, 1780;
- 10fM. Schnürch, N. Dastbaravardeh, M. Ghobrial, B. Mrozek, M. D. Mihovilovic, Curr. Org. Chem. 2011, 15, 2694;
- 10gA. W. Wendlandt, A. M. Suess, S. S. Stahl, Angew. Chem. 2011, 123, 11256;
10.1002/ange.201103945 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11062;
- 10hC. Zhang, C.-H. Tang, N. Jiao, Chem. Soc. Rev. 2012, 41, 3464. For selected recent examples, see:
- 10iP. S. Baran, M. P. DeMartino, Angew. Chem. 2006, 118, 7241;
10.1002/ange.200603024 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7083;
- 10jT. Hamada, X. Ye, S. S. Stahl, J. Am. Chem. Soc. 2008, 130, 833;
- 10kG. Brasche, S. L. Buchwald, Angew. Chem. 2008, 120, 1958;
10.1002/ange.200705420 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1932;
- 10lS. Ueda, H. Nagasawa, Angew. Chem. 2008, 120, 6511; Angew. Chem. Int. Ed. 2008, 47, 6411;
- 10mO. Baslé, C.-J. Li, Chem. Commun. 2009, 4124;
- 10nS. Ueda, H. Nagasawa, J. Am. Chem. Soc. 2009, 131, 15080;
- 10oS. Ueda, H. Nagasawa, J. Org. Chem. 2009, 74, 4272;
- 10pC. Zhang, N. Jiao, J. Am. Chem. Soc. 2010, 132, 28;
- 10qC. Zhang, N. Jiao, Angew. Chem. 2010, 122, 6310; Angew. Chem. Int. Ed. 2010, 49, 6174;
- 10rH. Wang, Y. Wang, C. Peng, J. Zhang, Q. Zhu, J. Am. Chem. Soc. 2010, 132, 13217;
- 10sH. Wang, Y. Wang, D. Liang, L. Liu, J. Zhang, Q. Zhu, Angew. Chem. 2011, 123, 5796; Angew. Chem. Int. Ed. 2011, 50, 5678;
- 10tL. M. Huffman, A. Casitas, M. Font, M. Canta, M. Costas, X. Ribas, S. S. Stahl, Chem. Eur. J. 2011, 17, 10643;
- 10uL. Zhang, Z.-H. Liu, H.-Q. Li, G.-C. Fang, B.-D. Barry, T. A. Belay, X.-H. Bi, Q. Liu, Org. Lett. 2011, 13, 6536;
- 10vK. K. Toh, Y.-F. Wang, E. P. J. Ng, S. Chiba, J. Am. Chem. Soc. 2011, 133, 13942;
- 10wY.-F. Wang, X. Zhu, S. Chiba, J. Am. Chem. Soc. 2012, 134, 3679;
- 10xZ.-J. Xu, C. Zhang, N. Jiao, Angew. Chem. 2012, 124, 11529; Angew. Chem. Int. Ed. 2012, 51, 11367.
- 11
- 11aY.-F. Wang, H. Chen, X. Zhu, S. Chiba, J. Am. Chem. Soc. 2012, 134, 11980;
- 11bH. Chen, S. Sanjaya, Y.-F. Wang, S. Chiba, Org. Lett. 2013, 15, 212.
- 12E. M. Simmons, J. F. Hartwig, Angew. Chem. 2012, 124, 3120;
10.1002/ange.201107334 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3066.
- 13
- 13aH. Furuta, H. Maeda, A. Osuka, J. Am. Chem. Soc. 2000, 122, 803;
- 13bX. Ribas, D. A. Jackson, B. Donnadieu, J. Mahía, T. Parella, R. Xifra, B. Hedman, K. O. Hodgson, A. Llobet, T. D. P. Stack, Angew. Chem. 2002, 114, 3117;
10.1002/1521-3757(20020816)114:16<3117::AID-ANGE3117>3.0.CO;2-U Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2991;10.1002/1521-3773(20020816)41:16<2991::AID-ANIE2991>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 13cH. Maeda, A. Osuka, H. Furuta, J. Am. Chem. Soc. 2003, 125, 15690;
- 13dR. Xifra, X. Ribas, A. Llobet, A. Poater, M. Duran, M. Solà, T. D. P. Stack, J. Benet-Buchholz, B. Donnadieu, J. Mahía, T. Parella, Chem. Eur. J. 2005, 11, 5146;
- 13eM. Pawlicki, I. Kanska, L. Latos-Graźynski, Inorg. Chem. 2007, 46, 6575;
- 13fL. M. Huffman, S. S. Stahl, J. Am. Chem. Soc. 2008, 130, 9196;
- 13gB. Yao, D.-X. Wang, Z.-T. Huang, M.-X. Wang, Chem. Commun. 2009, 2899;
- 13hA. E. King, L. M. Huffman, A. Casitas, M. Costas, X. Ribas, S. S. Stahl, J. Am. Chem. Soc. 2010, 132, 1147;
- 13iA. E. King, L. M. Huffman, A. Casitas, M. Costas, X. Ribas, S. S. Stahl, J. Am. Chem. Soc. 2010, 132, 12068;
- 13jX. Ribas, C. Calle, A. Poater, A. Casitas, L. Gómez, R. Xifra, T. Parella, J. Benet-Buchholz, A. Schweiger, G. Mitrikas, M. Solà, A. Llobet, T. D. P. Stack, J. Am. Chem. Soc. 2010, 132, 12299;
- 13kA. M. Suess, M. Z. Ertem, C. J. Cramer, S. S. Stahl, J. Am. Chem. Soc. 2013, 135, 9797.
- 14While 3-chloro-2,2-dimethyl-N-(quinolin-8-yl)propanamide (1 u) was subjected to the standard reaction conditions, 2 a was observed as the product. This result indicates that a sequential chlorination amidation process is also possible.
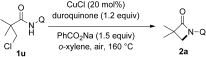
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.




