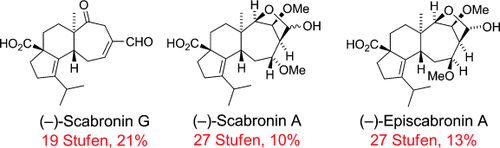
Total Syntheses of (−)-Scabronines G and A, and (−)-Episcabronine A†
Yu Kobayakawa
Department of Chemistry and Biochemistry, Faculty of Science and Engineering, Waseda University, 3-4-1 Ohkubo, Shinjuku-ku, Tokyo 169-8555 (Japan) http://www.chem.waseda.ac.jp/nakada/
Search for more papers by this authorCorresponding Author
Prof. Masahisa Nakada
Department of Chemistry and Biochemistry, Faculty of Science and Engineering, Waseda University, 3-4-1 Ohkubo, Shinjuku-ku, Tokyo 169-8555 (Japan) http://www.chem.waseda.ac.jp/nakada/
Department of Chemistry and Biochemistry, Faculty of Science and Engineering, Waseda University, 3-4-1 Ohkubo, Shinjuku-ku, Tokyo 169-8555 (Japan) http://www.chem.waseda.ac.jp/nakada/Search for more papers by this authorYu Kobayakawa
Department of Chemistry and Biochemistry, Faculty of Science and Engineering, Waseda University, 3-4-1 Ohkubo, Shinjuku-ku, Tokyo 169-8555 (Japan) http://www.chem.waseda.ac.jp/nakada/
Search for more papers by this authorCorresponding Author
Prof. Masahisa Nakada
Department of Chemistry and Biochemistry, Faculty of Science and Engineering, Waseda University, 3-4-1 Ohkubo, Shinjuku-ku, Tokyo 169-8555 (Japan) http://www.chem.waseda.ac.jp/nakada/
Department of Chemistry and Biochemistry, Faculty of Science and Engineering, Waseda University, 3-4-1 Ohkubo, Shinjuku-ku, Tokyo 169-8555 (Japan) http://www.chem.waseda.ac.jp/nakada/Search for more papers by this authorThis work was financially supported in part by the Grant-in-Aid for Scientific Research on Innovative Areas. 2105 Organic Synthesis Based on Reaction Integration: Development of New Methods and Creation of New Substances and for Scientific Research (B) (25293003) by the MEXT, and a Waseda University Grant for Special Research Projects. We are grateful to Prof. T. Ohta (Kanazawa University) for sending us copies of 1H and 13C NMR spectra. The fellowship for young scientists to Y.K. from JSPS (5515) is also gratefully acknowledged.
Graphical Abstract
Eine hoch stereoselektive Reaktionskaskade aus oxidativer Desaromatisierung und intramolekularer Diels-Alder-Reaktion mit inversem Elektronenbedarf steht im Mittelpunkt einer Totalsynthese von (−)-Scabronin G, während die erste Totalsynthese von (−)-Scabronin A auf einer hoch stereoselektiven Kaskade aus Oxa-Michael-Reaktion, Protonierung und Acetalisierung beruht. Bei der ersten Totalsynthese von (−)-Episcabronin A wurde analog verfahren.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201303224_sm_miscellaneous_information.pdf18.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Scabronine A:
- 1aT. Ohta, T. Kita, N. Kobayashi, Y. Obara, N. Nakahata, Y. Ohizumi, Y. Takaya, Y. Oshima, Tetrahedron Lett. 1998, 39, 6229–6232; Scabronines B–F:
- 1bT. Kita, Y. Takaya, Y. Oshima, T. Ohta, K. Aizawa, T. Hirano, T. Inakuma, Tetrahedron 1998, 54, 11877–11886; Scabronine G:
- 1cY. Obara, H. Kobayashi, T. Ohta, Y. Ohizumi, N. Nakahata, Mol. Pharmacol. 2001, 59, 1287–1297; For episcabronine A:
- 1dT. Ohta, H. Kobayashi, S. Hosoi, F. Kiuchi, T. Kita, Y. Takaya, Y. Oshima, Y. Obara, N. Nakahata, Y. Ohizumi, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 1998, 40, 341–346.
- 2
- 2aY. Obara, N. Nakahata, T. Kita, Y. Takaya, H. Kobayashi, S. Hosoi, F. Kiuchi, T. Ohta, Y. Oshima, Y. Ohizumi, Eur. J. Pharmacol. 1999, 370, 79–84;
- 2bX.-W. Shi, L. Liu, J.-M. Gao, A.-L. Zhang, Eur. J. Med. Chem. 2011, 46, 3112–3117.
- 3
- 3aX. He, K. C. Garcia, Science 2004, 304, 870–875;
- 3bM. V. Sofroniew, C. L. Howe, W. C. Mobley, Annu. Rev. Neurosci. 2001, 24, 1217–1281;
- 3cR. M. Lindsay, J. Neurosci. 1988, 8, 2394–2405.
- 4F. Hefti, Annu. Rev. Pharmacol. Toxicol. 1997, 37, 239–267.
- 5
- 5aS. Rosenberg, Annu. Rep. Med. Chem. 1992, 27, 41–48;
- 5bS. Carswell, Exp. Neurol. 1993, 124, 36–42;
- 5cC. F. Bigge, P. A. Boxer, Annu. Rep. Med. Chem. 1994, 29, 13–22;
- 5dS. S. Riaz, D. R. Tomlinson, Prog. Neurobiol. 1996, 49, 125–143;
- 5eJ. M. Conner, M. H. Tuszynski, Ment. Retard Dev. Dis. 1998, 4, 212–222;
- 5fP. A. Dijkhuizen, J. Verhaagen, Neuro. Res. Commun. 1999, 24, 1–10;
- 5gH. U. Saragovi, K. Burgess, Expert Opin. Ther. Pat. 1999, 9, 737–751;
- 5hD. Kirik, B. Georgievska, A. Bjorklund, Nat. Neurosci. 2004, 7, 105–110.
- 6S. J. Pollack, S. J. Harper, Curr. Drug Targets: CNS Neurol. Disord. 2002, 1, 59–80.
- 7For a review see:
- 7aD. L. Wright, C. R. Whitehead, Org. Prep. Proced. Int. 2000, 32, 307–330;
- 7bJ. A. Enquist, Jr., B. M. Stoltz, Nat. Prod. Rep. 2009, 26, 661–680.
- 8Synthetic studies:
- 8aW. A. Ayer, D. E. Ward, L. M. Browne, L. T. J. Delbaere, Y. Hoyano, Can. J. Chem. 1981, 59, 2665–2672;
- 8bD. E. Ward, Can. J. Chem. 1987, 65, 2380–2384;
- 8cK. R. Dahnke, L. A. Paquette, J. Org. Chem. 1994, 59, 885–899;
- 8dE. Piers, K. L. Cook, Chem. Commun. 1996, 1879–1880;
- 8eP. Magnus, L. Shen, Tetrahedron 1999, 55, 3553–3560;
- 8fD. L. Wright, C. R. Whitehead, E. H. Sessions, I. Ghiviriga, D. A. Frey, Org. Lett. 1999, 1, 1535–1538;
- 8gK. Takeda, D. Nakane, M. Takeda, Org. Lett. 2000, 2, 1903–1905;
- 8hP. A. Wender, F. C. Bi, M. A. Brodney, F. Gosselin, Org. Lett. 2001, 3, 2105–2108;
- 8iC. Thominiaux, A. Chiaroni, D. Desmaele, Tetrahedron Lett. 2002, 43, 4107–4110.
- 9Total synthesis: (±)-allocyathin B2:
- 9aB. B. Snider, N. H. Vo, S. V. O’Neil, B. M. Foxman, J. Am. Chem. Soc. 1996, 118, 7644–7645;
- 9bB. B. Snider, N. H. Vo, S. V. O’Neil, J. Org. Chem. 1998, 63, 4732–4740;
- 9cM. Tori, N. Toyoda, M. Sono, J. Org. Chem. 1998, 63, 306–313; (±)-Sarcodonin G:
- 9dE. Piers, M. Gilbert, K. L. Cook, Org. Lett. 2000, 2, 1407–1410; (±)-allocyathin B3:
- 9eD. E. Ward, Y. Gai, Q. Qiao, Org. Lett. 2000, 2, 2125–2127;
- 9fD. E. Ward, Y. Gai, Q. Qiao, J. Shen, Can. J. Chem. 2004, 82, 254–267; (+)-Allocyathin B2:
- 9gM. Takano, A. Umino, M. Nakada, Org. Lett. 2004, 6, 4897–4900;
- 9hB. M. Trost, L. Dong, G. M. Schroeder, J. Am. Chem. Soc. 2005, 127, 2844–2845;
- 9iB. M. Trost, L. Dong, G. M. Schroeder, J. Am. Chem. Soc. 2005, 127, 10259–10268; (+)-cyanthiwigin U:
- 9jM. W. B. Pfeiffer, A. J. Phillips, J. Am. Chem. Soc. 2005, 127, 5334–5335; (−)-erinacine B:
- 9kH. Watanabe, M. Takano, A. Umino, T. Ito, H. Ishikawa, M. Nakada, Org. Lett. 2007, 9, 359–362; (−)-cyathin A3:
- 9lD. E. Ward, J. Shen, Org. Lett. 2007, 9, 2843–2846; (−)-Erinacine E:
- 9mH. Watanabe, M. Nakada, J. Am. Chem. Soc. 2008, 130, 1150–1151; (−)-cyanthiwigin F:
- 9nJ. A. Enquist Jr., B. M. Stoltz, Nature 2008, 453, 1228–1231; (±)-cyathin A3 and B2:
- 9oK. Kim, J. K. Cha, Angew. Chem. 2009, 121, 5438–5440; Angew. Chem. Int. Ed. 2009, 48, 5334–5336; (+)-cyrneine A:
- 9pE. Elamparuthi, C. Fellay, M. Neuburger, K. Gademann, Angew. Chem. 2012, 124, 4147–4149;
10.1002/ange.201200515 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4071–4073.
- 10
- 10aS. P. Waters, Y. Tian, Y.-M. Li, S. J. Danishefsky, J. Am. Chem. Soc. 2005, 127, 13514–13515;
- 10bN. Kanoh, K. Sakanishi, E. Iimori, K. Nishimura, Y. Iwabuchi, Org. Lett. 2011, 13, 2864–2867.
- 11Our efforts on the total synthesis of scabronine A: H. Watanabe, M. Nakada, Tetrahedron Lett. 2008, 49, 1518–1522.
- 12Reviews of masked o-benzoquinones (MOBs) in organic synthesis:
- 12aC.-C. Liao, R. K. Peddinti, Acc. Chem. Res. 2002, 35, 856–866;
- 12bD. Magdziak, S. J. Meek, T. R. R. Pettus, Chem. Rev. 2004, 104, 1383–1430;
- 12cC.-C. Liao, Pure Appl. Chem. 2005, 77, 1221–1234;
- 12dS. P. Roche, J. A. Porco, Jr., Angew. Chem. 2011, 123, 4154–4179;
10.1002/ange.201006017 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4068–4093.
- 13Precedents of the intramolecular IEDDA reaction cascade:
- 13aS. P. Cook, C. Gaul, S. J. Danishefsky, Tetrahedron Lett. 2005, 46, 843–847;
- 13bS. P. Cook, A. Polara, S. J. Danishefsky, J. Am. Chem. Soc. 2006, 128, 16440–16441;
- 13cA. Polara, S. P. Cook, S. J. Danishefsky, Tetrahedron Lett. 2008, 49, 5906–5908;
- 13dG. Y. C. Leung, H. Li, Q.-Y. Toh, A. M.-Y. Ng, R. J. Sum, J. E. Bandow, D. Y.-K. Chen, Eur. J. Org. Chem. 2011, 183–196.
- 14R. Tello-Aburto, A. M. Harned, Org. Lett. 2009, 11, 3998–4000.
- 15T. S. Kaufman, J. Chem. Soc. Perkin Trans. 1 1996, 2497–2505.
- 16K. C. Nicolaou, K. P. Cole, M. O. Frederick, R. J. Aversa, R. M. Denton, Angew. Chem. 2007, 119, 9031–9035;
10.1002/ange.200703742 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8875–8879.
- 17
- 17aS. Hashiguchi, A. Fujii, J. Takehara, T. Ikariya, R. Noyori, J. Am. Chem. Soc. 1995, 117, 7562–7563;
- 17bK.-J. Haack, S. Hashiguchi, A. Fujii, T. Ikariya, R. Noyori, Angew. Chem. 1997, 109, 297–300;
10.1002/ange.19971090333 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 285–288.
- 18
- 18aP. Knochel, M. C. P. Yeh, S. C. Berk, J. Talbert, J. Org. Chem. 1988, 53, 2390–2392;
- 18bP. Knochel, W. Dohle, N. Gommermann, F. F. Kneisel, F. Kopp, T. Korn, I. Sapountzis, V. A. Vu, Angew. Chem. 2003, 115, 4438–4456;
10.1002/ange.200300579 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4302–4320.
- 19The intramolecular IEDDA reaction at elevated temperature produces unknown by-products probably owing to the remaining PIDA.
- 20CCDC 934431 (14) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif..
- 21R. W. Heidebrecht, Jr., B. Gulledge, S. F. Martin, Org. Lett. 2010, 12, 2492–2495.
- 22K. C. Nicolaou, V. A. Adsool, C. R. H. Hale, Org. Lett. 2010, 12, 1552–1555.
- 23M. Scholl, S. Ding, C. W. Lee, R. H. Grubbs, Org. Lett. 1999, 1, 953–956.
- 24E. J. Corey, R. K. Bakshi, S. Shibata, J. Am. Chem. Soc. 1987, 109, 5551–5553.
- 25For the intermolecular oxa-Michael reaction in natural product synthesis, see:
- 25aM. J. Ford, J. G. Knight, S. V. Ley, S. Vile, Synlett 1990, 331–332;
- 25bS. V. Ley, A. Armstrong, D. Díez-Martín, M. J. Ford, P. Grice, J. G. Knight, H. C. Kolb, A. Madin, C. A. Marby, S. Mukherjee, A. N. Shaw, A. M. Z. Slawin, S. Vile, A. D. White, D. J. Williams, M. Woods, J. Chem. Soc. Perkin Trans. 1 1991, 667–692.
- 26The [α]D value of synthesized 1 {
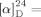 −40.9 (c=0.74, MeOH)} is different from the reported value {
−40.9 (c=0.74, MeOH)} is different from the reported value {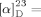 −15.0 (c=0.92, MeOH)[1a]}. This difference may be attributed to varying [α]D contributions by the C15 isomers of 1, a characteristic which was similarly noted for 19 (see the Supporting information).
−15.0 (c=0.92, MeOH)[1a]}. This difference may be attributed to varying [α]D contributions by the C15 isomers of 1, a characteristic which was similarly noted for 19 (see the Supporting information).
- 27The cascade reactions in this work could suggest that (−)-scabronine A and (−)-episcabronine A are artifacts because these compounds were isolated from the methanol extract of Sarcodon scabrosus. We are now investigating possibility of their formation during the isolation.
- 28S. Suga, D. Yamada, J. Yoshida, Chem. Lett. 2010, 39, 404–406.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.