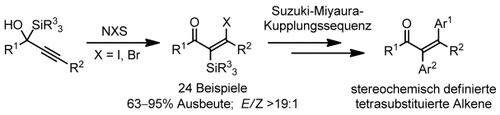
Stereoselective Synthesis of Tetrasubstituted Olefins through a Halogen-Induced 1,2-Silyl Migration†
Dr. Nicholas T. Barczak
Department of Chemistry, Colorado State University, 1872 Campus Delivery, Fort Collins, CO 80523 (USA)
Search for more papers by this authorDouglas A. Rooke
Department of Chemistry, Colorado State University, 1872 Campus Delivery, Fort Collins, CO 80523 (USA)
Search for more papers by this authorZachary A. Menard
Department of Chemistry, Colorado State University, 1872 Campus Delivery, Fort Collins, CO 80523 (USA)
Search for more papers by this authorCorresponding Author
Prof. Eric M. Ferreira
Department of Chemistry, Colorado State University, 1872 Campus Delivery, Fort Collins, CO 80523 (USA)
Department of Chemistry, Colorado State University, 1872 Campus Delivery, Fort Collins, CO 80523 (USA)Search for more papers by this authorDr. Nicholas T. Barczak
Department of Chemistry, Colorado State University, 1872 Campus Delivery, Fort Collins, CO 80523 (USA)
Search for more papers by this authorDouglas A. Rooke
Department of Chemistry, Colorado State University, 1872 Campus Delivery, Fort Collins, CO 80523 (USA)
Search for more papers by this authorZachary A. Menard
Department of Chemistry, Colorado State University, 1872 Campus Delivery, Fort Collins, CO 80523 (USA)
Search for more papers by this authorCorresponding Author
Prof. Eric M. Ferreira
Department of Chemistry, Colorado State University, 1872 Campus Delivery, Fort Collins, CO 80523 (USA)
Department of Chemistry, Colorado State University, 1872 Campus Delivery, Fort Collins, CO 80523 (USA)Search for more papers by this authorColorado State University is acknowledged for the support of our program. Eli Lilly is gratefully acknowledged for a graduate research fellowship to D.A.R.
Graphical Abstract
Silicium auf Wanderschaft: Bei der stereoselektiven Bildung von hoch substituierten α-Silyl-β-halogenenonen induziert die elektrophile Aktivierung des Ausgangsalkins durch N-Halogensuccinimide eine anti-selektive Verschiebung der Silylgruppe (siehe Schema). Die resultierenden Enone können leicht und unter Erhaltung der Konfiguration in Alkene mit vier Kohlenstoffsubstituenten umgewandelt werden.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201303007_sm_miscellaneous_information.pdf4.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. B. Flynn, W. W. Ogilvie, Chem. Rev. 2007, 107, 4698–4745.
- 2For examples, see:
- 2aA. Nandakumar, P. T. Perumal, Org. Lett. 2013, 15, 382–385;
- 2bR. Daik, W. J. Feast, A. S. Batsanov, J. A. K. Howard, New J. Chem. 1998, 22, 1047–1049;
- 2cW. R. Browne, M. M. Pollard, B. de Lange, A. Meetsma, B. L. Feringa, J. Am. Chem. Soc. 2006, 128, 12412–12413;
- 2dJ. Vicario, M. Walko, A. Meetsma, B. L. Feringa, J. Am. Chem. Soc. 2006, 128, 5127–5135;
- 2eN. N. P. Moonen, C. Boudon, J.-P. Gisselbrecht, P. Seiler, M. Gross, F. Diederich, Angew. Chem. 2002, 114, 3170–3173;
10.1002/1521-3757(20020816)114:16<3170::AID-ANGE3170>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3044–3047.10.1002/1521-3773(20020816)41:16<3044::AID-ANIE3044>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 3For select examples, see:
- 3aJ. R. Calvin, M. O. Frederick, D. L. T. Laird, J. R. Remacle, S. A. May, Org. Lett. 2012, 14, 1038–1041;
- 3bK. Friedrich, R. Zimmer, J. Prakt. Chem. 1998, 340, 757–759;
- 3cS. F. Oliver, K. Högenauer, O. Simic, A. Antonello, M. D. Smith, S. V. Ley, Angew. Chem. 2003, 115, 6178–6182; Angew. Chem. Int. Ed. 2003, 42, 5996–6000.
- 4For select examples of intramolecular olefinations, see:
- 4aG. A. Kraus, T. Goronga, Synthesis 2007, 1765–1767;
- 4bB. Prabhudas, D. L. J. Clive, Angew. Chem. 2007, 119, 9455–9457;
10.1002/ange.200703022 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 9295–9297;
- 4cS. P. Andrews, M. M. Tait, M. Ball, S. V. Ley, Org. Biomol. Chem. 2007, 5, 1427–1436;
- 4dJ. H. Frederich, P. G. Harran, J. Am. Chem. Soc. 2013, 135, 3788–3791;
- 4eM. M. Ott, R. D. Little, J. Org. Chem. 1997, 62, 1610–1616;
- 4fI. C. Stewart, T. Ung, A. A. Pletnev, J. M. Berlin, R. H. Grubbs, Y. Schrodi, Org. Lett. 2007, 9, 1589–1592;
- 4gD. Rost, M. Porta, S. Gessler, S. Blechert, Tetrahedron Lett. 2008, 49, 5968–5971;
- 4hV. Sashuk, L. H. Peeck, H. Plenio, Chem. Eur. J. 2010, 16, 3983–3993.
- 5For select reviews of alkyne carbometalation, see:
- 5aJ. F. Normant, A. Alexakis, Synthesis 1981, 841–870;
- 5bA. G. Fallis, P. Forgione, Tetrahedron 2001, 57, 5899–5913;
- 5cE.-i. Negishi, G. Wang, H. Rao, Z. Xu, J. Org. Chem. 2010, 75, 3151–3182.
- 6For related methods of halogen-derived additions to alkynes and variants thereof, see,
- 6aG. F. Koser, L. Rebrovic, R. H. Wettach, J. Org. Chem. 1981, 46, 4324–4326;
- 6bM. G. Suero, E. D. Baykle, B. S. L. Collins, M. J. Gaunt, J. Am. Chem. Soc. 2013, 135, 5332–5335;
- 6cT. M. Kasumov, N. S. Pirguliyev, V. K. Brel, Y. K. Grishin, N. S. Zefirov, P. J. Stang, Tetrahedron 1997, 53, 13139–13148.
- 7
- 7aD. A. Rooke, E. M. Ferreira, J. Am. Chem. Soc. 2010, 132, 11926–11928;
- 7bD. A. Rooke, E. M. Ferreira, Angew. Chem. 2012, 124, 3279–3284;
10.1002/ange.201108714 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3225–3230;
- 7cD. A. Rooke, E. M. Ferreira, Org. Lett. 2012, 14, 3328–3331.
- 8For a recent review on the synthesis of vinylsilanes, see, D. S. W. Lim, E. A. Anderson, Synthesis 2012, 983–1010.
- 9For an interesting approach to the synthesis of vinylsilanes using a “silyl-Heck” reaction, see,
- 9aH. Yamashita, T.-a. Kobayashi, T. Hayashi, M. Tanaka, Chem. Lett. 1991, 761–762;
- 9bJ. R. McAtee, S. E. S. Martin, D. T. Ahneman, K. A. Johnson, D. A. Watson, Angew. Chem. 2012, 124, 3723–3727;
10.1002/ange.201200060 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3663–3667.
- 10For additional studies on the reactivity and utility of α-hydroxypropargylsilanes, see:
- 10aM. Kato, I. Kuwajima, Bull. Chem. Soc. Jpn. 1984, 57, 827–830;
- 10bH. J. Reich, E. K. Eisenhart, R. E. Olson, M. J. Kelly, J. Am. Chem. Soc. 1986, 108, 7791–7800;
- 10cB. Mergardt, K. Weber, G. Adiwidjaja, E. Schaumann, Angew. Chem. 1991, 103, 1679–1680; Angew. Chem. Int. Ed. Engl. 1991, 30, 1687–1688;
- 10dT. E. Reynolds, A. R. Bharadwaj, K. A. Scheidt, J. Am. Chem. Soc. 2006, 128, 15382–15383;
- 10eT. E. Reynolds, K. A. Scheidt, Angew. Chem. 2007, 119, 7952–7955;
10.1002/ange.200702818 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7806–7809.
- 11
- 11aJ. Enda, I. Kuwajima, J. Chem. Soc. Chem. Commun. 1984, 1589;
- 11bK. Sakaguchi, M. Higashino, Y. Ohfune, Tetrahedron 2003, 59, 6647–6658.
- 12For recent reviews of reactions of alkynes using electrophilc iodine, see:
- 12aA. Palisse, S. F. Kirsch, Org. Biomol. Chem. 2012, 10, 8041–8047;
- 12bY. Yamamoto, I. D. Gridnev, N. T. Patil, T. Jin, Chem. Commun. 2009, 5075–5087.
- 13For an elegant approach to β-haloenones based on alkyne activation, see, J. Sun, S. A. Kozmin, J. Am. Chem. Soc. 2005, 127, 13512–13513.
- 14NCS did induce similar reactivity at more elevated temperatures in protic solvents (50 °C in EtOH), likely enabled through mild H-bond activation of the succinimide reagent.
- 15The stereochemistry of the migration was confirmed in several cases by NOE analysis. See the Supporting Information for details.
- 16The corresponding ynone was observed as the sole product from this attempted iodosilylation.
- 17Considering the impact of the halogen atom in entry 3 compared to the analogous iodination (Table 2, entry 3), the ynone compound seen in iodination likely arises through a rapid trans elimination of the silicon group and iodide species from the targeted enone product.
- 18
- 18aA. Alimardanov, E.-i. Negishi, Tetrahedron Lett. 1999, 40, 3839–3842;
- 18bTemperature control was crucial for the success of this transformation. When the reactions were performed at 0 °C, approximately 1:1 mixtures of alkene isomers were observed.
- 19For select examples of Suzuki–Miyaura cross couplings to synthesize stereodefined tetrasubstituted alkenes, see:
- 19aA. N. Thadani, V. H. Rawal, Org. Lett. 2002, 4, 4317–4320;
- 19bK. Itami, T. Kamei, J.-i. Yoshida, J. Am. Chem. Soc. 2003, 125, 14670–14671;
- 19cM. Suginome, A. Yamamoto, M. Murakami, Angew. Chem. 2005, 117, 2432–2434;
10.1002/ange.200462961 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2380–2382;
- 19dC. Zhou, R. C. Larock, J. Org. Chem. 2005, 70, 3765–3777;
- 19eM. Shimizu, C. Nakamaki, K. Shimono, M. Schelper, T. Kurahashi, T. Hiyama, J. Am. Chem. Soc. 2005, 127, 12506–12507;
- 19fY. Nishihara, M. Miyasaka, M. Okamoto, H. Takahashi, E. Inoue, K. Tanemura, K. Takagi, J. Am. Chem. Soc. 2007, 129, 12634–12635;
- 19gW. You, Y. Li, M. K. Brown, Org. Lett. 2013, 15, 1610–1613.
- 20Although we predicted a direct Hiyama cross coupling with an α-silylenone or a variant would be possible, several conditions explored were unsuccessful, presumably owing to the instability of α-silylenones with the nucleophilic fluoride reagents that are generally required for transmetalation. For a discussion, see Ref. [7c].
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.