Iridium-Catalyzed Enantioselective Allylic Alkynylation†
James Y. Hamilton
ETH Zürich, HCI H335, 8093 Zürich (Switzerland)
Search for more papers by this authorDr. David Sarlah
ETH Zürich, HCI H335, 8093 Zürich (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Erick M. Carreira
ETH Zürich, HCI H335, 8093 Zürich (Switzerland)
ETH Zürich, HCI H335, 8093 Zürich (Switzerland)Search for more papers by this authorJames Y. Hamilton
ETH Zürich, HCI H335, 8093 Zürich (Switzerland)
Search for more papers by this authorDr. David Sarlah
ETH Zürich, HCI H335, 8093 Zürich (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Erick M. Carreira
ETH Zürich, HCI H335, 8093 Zürich (Switzerland)
ETH Zürich, HCI H335, 8093 Zürich (Switzerland)Search for more papers by this authorWe are grateful to the ETH Zürich and the Swiss National Science Foundation for financial support.
Graphical Abstract
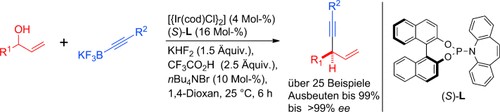
Ohne Abgangsgruppe: Ein Ir-Komplex mit P,Olefin-Ligand katalysiert die direkte enantioselektive allylische Alkinylierung sekundärer Allylalkohole mit Kaliumalkinyltrifluoroboraten. Das leicht durchführbare und robuste Verfahren, das hohe Enantioselektivitäten und Ausbeuten erzielt, wurde in der Synthese des GPR40-Rezeptoragonisten AMG 837 angewendet. cod=1,5-Cyclooctadien.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201302731_sm_miscellaneous_information.pdf5.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Comprehensive Asymmetric Catalysis (Eds.: ), Springer, Berlin, 1999, and Comprehensive Asymmetric Catalysis (Eds.: ), Springer, Berlin, 2003 (For Supplement I);
- 1b Catalytic Asymmetric Synthesis (Ed.: ), Wiley, New York, 2000;
10.1002/0471721506 Google Scholar
- 1c New Frontiers in Asymmetric Catalysis (Eds.: ), Wiley, Hoboken, 2007.
- 2
- 2a Modern Acetylene Chemistry (Eds.: ), VCH, Weinheim, 1995;
- 2b Acetylene Chemistry: Chemistry, Biology, and Material Science (Eds.: ), VCH, Weinheim, 2005.
- 3For recent reviews, see:
- 3aB. M. Trost, A. H. Weiss, Adv. Synth. Catal. 2009, 351, 963;
- 3bG. Lu, Y. M. Li, X. S. Li, A. S. C. Chan, Coord. Chem. Rev. 2005, 249, 1736;
- 3cP. G. Cozzi, R. Hilgraf, N. Zimmermann, Eur. J. Org. Chem. 2004, 4095;
- 3dL. Pu, Tetrahedron 2003, 59, 9873.
- 4For examples, see:
- 4aT. Nishimura, X.-X. Guo, T. Hayashi, Chem. Asian J. 2008, 3, 1505;
- 4bM. Shirakura, M. Suginome, Angew. Chem. 2010, 122, 3915;
10.1002/ange.201001188 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 3827;
- 4cB.-M. Fan, Q.-J. Yang, J. Hu, C.-L. Fan, S.-F. Li, L. Yu, C. Huang, W.-W. Tsang, F.-Y. Kwong, Angew. Chem. 2012, 124, 7941; Angew. Chem. Int. Ed. 2012, 51, 7821; for sequential hydroalkynylation/asymmetric 1,4-reduction, see:
- 4dB. M. Trost, B. R. Taft, J. T. Masters, J.-P. Lumb, J. Am. Chem. Soc. 2011, 133, 8502.
- 5“Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis”: Topics in Organometallic Chemistry, Vol. 38 (Ed.: ), Springer, Heidelberg, 2012.
- 6For recent reviews, see:
- 6aS. R. Harutyunyan, T. den Hartog, K. Geurts, A. J. Minnaard, B. L. Feringa, Chem. Rev. 2008, 108, 2824;
- 6bZ. Lu, S. Ma, Angew. Chem. 2008, 120, 264; Angew. Chem. Int. Ed. 2008, 47, 258;
- 6cA. Alexakis, J. E. Bäckvall, N. Krause, O. Pamies, M. Dieguez, Chem. Rev. 2008, 108, 2796;
- 6dC. A. Falciola, A. Alexakis, Eur. J. Org. Chem. 2008, 3765; for allylic substitution with alkylboranes, see:
- 6eY. Shido, M. Yoshida, M. Tanabe, H. Ohmiya, M. Sawamura, J. Am. Chem. Soc. 2012, 134, 18573.
- 7J. A. Dabrowski, F. Gao, A. H. Hoveyda, J. Am. Chem. Soc. 2011, 133, 4778.
- 8H. Li, A. Alexakis, Angew. Chem. 2012, 124, 1079; Angew. Chem. Int. Ed. 2012, 51, 1055.
- 9For other asymmetric allylic alkylation reports employing enyne electrophiles, see:
- 9aB. M. Trost, S. Hildbrand, K. Dogra, J. Am. Chem. Soc. 1999, 121, 10416;
- 9bM. A. Kacprzynski, A. H. Hoveyda, J. Am. Chem. Soc. 2004, 126, 10676.
- 10For seminal contributions, see:
- 10aR. Takeuchi, M. Kashio, Angew. Chem. 1997, 109, 268;
10.1002/ange.19971090320 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 263;
- 10bJ. P. Janssen, G. Helmchen, Tetrahedron Lett. 1997, 38, 8025;
- 10cT. Ohmura, J. F. Hartwig, J. Am. Chem. Soc. 2002, 124, 15164.
- 11
- 11aC. Defieber, M. A. Ariger, P. Moriel, E. M. Carreira, Angew. Chem. 2007, 119, 3200;
10.1002/ange.200700159 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3139;
- 11bM. Lafrance, M. Roggen, E. M. Carreira, Angew. Chem. 2012, 124, 3527;
10.1002/ange.201108287 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3470;
- 11cM. Roggen, E. M. Carreira, Angew. Chem. 2011, 123, 5683;
10.1002/ange.201007716 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 5568;
- 11dM. Roggen, E. M. Carreira, Angew. Chem. 2012, 124, 8780;
10.1002/ange.201202092 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 8652;
- 11eM. Schafroth, D. Sarlah, S. Krautwald, E. M. Carreira, J. Am. Chem. Soc. 2012, 134, 20276;
- 11fJ. Y. Hamilton, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc. 2013, 135, 994.
- 12For use of tetrabutylammonium salts in phase-transfer catalysis, see:
- 12aA. N. Thadani, R. A. Batey, Tetrahedron Lett. 2003, 44, 8051;
- 12bA. N. Thadani, R. A. Batey, Org. Lett. 2002, 4, 3827.
- 13For an example of the generation of HF in the organic phase when using a solid fluoride source, acid, and phase-transfer catalysis, see: G. Rothenberg, M. Royz, O. Arrad, Y. Sasson, J. Chem. Soc. Perkin Trans. 1 1999, 1491.
- 14M. Akerman, J. Houze, D. C. H. Lin, J. Liu, J. Luo, J. C. Medina, W. Qiu, J. D. Reagan, R. Sharma, S. J. Shuttleworth, Y. Sun, J. Zhang, L. Zhu, Int. Appl. PCT, WO 2005086661, 2005.
- 15For previous enantioselective approaches, see:
- 15aS. Cui, S. D. Walker, J. C. S. Woo, C. J. Borths, H. Mukherjee, M. J. Chen, M. M. Faul, J. Am. Chem. Soc. 2010, 132, 436;
- 15bJ. C. S. Woo, S. Cui, S. D. Walker, M. M. Faul, Tetrahedron 2010, 66, 4730;
- 15cR. Yazaki, N. Kumagai, M. Shibasaki, Org. Lett. 2011, 13, 952.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.