Pseudorotaxanes with Self-Sorted Sequence and Stereochemical Orientation†
Dr. Carmen Talotta
Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Search for more papers by this authorCorresponding Author
Dr. Carmine Gaeta
Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Carmine Gaeta, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Christoph A. Schalley, Institut für Chemie und Biochemie, Freie Universität, Takustrasse 3, 14195 Berlin (Germany)
Placido Neri, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Search for more papers by this authorZhenhui Qi
Institut für Chemie und Biochemie, Freie Universität, Takustrasse 3, 14195 Berlin (Germany)
Search for more papers by this authorCorresponding Author
Prof. Christoph A. Schalley
Institut für Chemie und Biochemie, Freie Universität, Takustrasse 3, 14195 Berlin (Germany)
Carmine Gaeta, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Christoph A. Schalley, Institut für Chemie und Biochemie, Freie Universität, Takustrasse 3, 14195 Berlin (Germany)
Placido Neri, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Search for more papers by this authorCorresponding Author
Prof. Placido Neri
Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Carmine Gaeta, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Christoph A. Schalley, Institut für Chemie und Biochemie, Freie Universität, Takustrasse 3, 14195 Berlin (Germany)
Placido Neri, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Search for more papers by this authorDr. Carmen Talotta
Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Search for more papers by this authorCorresponding Author
Dr. Carmine Gaeta
Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Carmine Gaeta, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Christoph A. Schalley, Institut für Chemie und Biochemie, Freie Universität, Takustrasse 3, 14195 Berlin (Germany)
Placido Neri, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Search for more papers by this authorZhenhui Qi
Institut für Chemie und Biochemie, Freie Universität, Takustrasse 3, 14195 Berlin (Germany)
Search for more papers by this authorCorresponding Author
Prof. Christoph A. Schalley
Institut für Chemie und Biochemie, Freie Universität, Takustrasse 3, 14195 Berlin (Germany)
Carmine Gaeta, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Christoph A. Schalley, Institut für Chemie und Biochemie, Freie Universität, Takustrasse 3, 14195 Berlin (Germany)
Placido Neri, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Search for more papers by this authorCorresponding Author
Prof. Placido Neri
Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Carmine Gaeta, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Christoph A. Schalley, Institut für Chemie und Biochemie, Freie Universität, Takustrasse 3, 14195 Berlin (Germany)
Placido Neri, Dipartimento di Chimica e Biologia and NANO_MATES Research Center, Università di Salerno, Via Giovanni Paolo II n. 132, 84084 Fisciano (Salerno) (Italy)
Search for more papers by this authorWe thank the Italian MIUR (PRIN 20109Z2XRJ_006) and the Deutsche Forschungsgemeinschaft (SFB 765) for funding. Z.Q. is grateful to the China Scholarship Council for a PhD fellowship.
Graphical Abstract
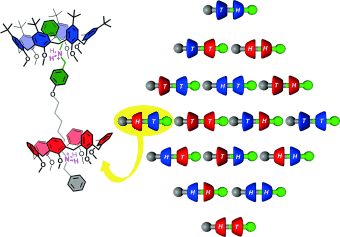
Partnerpräferenzen bei der Pseudorotaxanbildung wurden genutzt, um ein integratives selbstsortierendes System zu erstellen, das bezüglich Sequenz und Stereochemie simultan diskriminiert (siehe Bild). Es wurde gefunden, dass Calix[6]arene selektiv mit einer bevorzugten Orientierung auf Bisammonium-Achsen fädelten, selbst wenn die Strukturunterschiede zwischen den Bausteinen klein und entfernt von den Bindungszentren lokalisiert waren.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201301570_sm_miscellaneous_information.pdf15.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. M. Berg, J. L. Tymoczko, L. Stryer, Biochemistry, 6th ed., W. H. Freeman, New York, 2007;
- 1bN. A. Campbell, J. B. Reece, M. R. Taylor, E. J. Simon, J. L. Dickey, Biology: Concepts and Connections, 6th ed., Benjamin/Cummings, San Francisco, 2008.
- 2aJ. D. Watson, F. H. C. Crick, Nature 1953, 171, 737–738;
- 2bA. Yonath, Angew. Chem. 2010, 122, 4438–4453;
10.1002/ange.201001297 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 4340–4354.
- 3B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts, P. Walter, Molecular Biology of the Cell, 4th ed., Garland Science, New York, 2002.
- 4 Stereochemistry of Organic Compounds (Eds.: ), Wiley, New York, 1994.
- 5
- 5aA. D. Hershey, M. Chase, J. Gen. Physiol. 1952, 36, 39–56; for the role of RNA sequence isomers as start and stop codons, see: C. T. Caskey, R. Tompkins, E. Scolnick, T. Caryk, M. Nirenburg, Science 1968, 162, 135–138.
- 6
- 6aJ. E. Hein, D. G. Blackmond, Acc. Chem. Res. 2012, 45, 2045–2054;
- 6bM. Wu, S. I. Walker, P. G. Higgs, Int. J. Astrobiol. 2012, 12, 818–829;
- 6cA. S. Burton, J. C. Stern, J. E. Elsila, D. P. Glavin, J. P. Dworkin, Chem. Soc. Rev. 2012, 41, 5459–5472;
- 6dJ. E. Hein, E. Tse, D. G. Blackmond, Nat. Chem. 2011, 3, 704–706.
- 7For a definition of sequence isomers, see: A.-M. L. Fuller, D. A. Leigh, P. J. Lusby, J. Am. Chem. Soc. 2010, 132, 4954–4959.
- 8A. X. Wu, L. Isaacs, J. Am. Chem. Soc. 2003, 125, 4831–4835.
- 9For a recent review, see:
- 9aM. M. Safont-Sempere, G. Fernández, F. Würther, Chem. Rev. 2011, 111, 5784–5814; for some recent examples, see:
- 9bM. L. Saha, S. Pramanik, M. Schmittel, Chem. Commun. 2012, 48, 9459–9461;
- 9cE. Orentas, M. Lista, N.-T. Lin, N. Sakai, S. Matile, Nat. Chem. 2012, 4, 746–750;
- 9dC. Li, X. Shu, J. Li, J. Fan, Z. Chen, L. Weng, X. Jia, Org. Lett. 2012, 14, 4126–4129;
- 9eA. K. Mandal, P. Das, P. Mahato, S. Acharya, A. Das, J. Org. Chem. 2012, 77, 6789–6800;
- 9fA. S. Singh, S.-S. Sun, Chem. Commun. 2012, 48, 7392–7394;
- 9gR. C. Lirag, K. Osowska, O. Š. Miljanić, Org. Biomol. Chem. 2012, 10, 4847–4850;
- 9hM. Lal Saha, M. Schmittel, Org. Biomol. Chem. 2012, 10, 4651–4684;
- 9iM. Rancan, A. Dolmella, R. Seraglia, S. Orlandi, S. Quici, L. Armelao, Chem. Commun. 2012, 48, 3115–3117;
- 9jH. Gan, B. C. Gibb, Chem. Commun. 2012, 48, 1656–1658;
- 9kM. M. J. Smulders, A. Jiménez, J. R. Nitschke, Angew. Chem. 2012, 124, 6785–6789;
10.1002/ange.201202050 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6681–6685;
- 9lY. Yamauchi, M. Yoshizawa, M. Akita, M. Fujita, J. Am. Chem. Soc. 2010, 132, 960–966;
- 9mT. Murase, S. Horiuchi, M. Fujita, J. Am. Chem. Soc. 2010, 132, 7864–7865;
- 9nY. Rudzevich, V. Rudzevich, F. Klautzsch, C. A. Schalley, V. Böhmer Angew. Chem. 2009, 121, 3925–3929; Angew. Chem. Int. Ed. 2009, 48, 3867–3871; Angew. Chem. Int. Ed. 2009, 48, 3867–3871;
- 9oS. Ulrich, J.-M. Lehn, Chem. Eur. J. 2009, 15, 5640–5645;
- 9pS. Ulrich, J.-M. Lehn, J. Am. Chem. Soc. 2009, 131, 5546–5549;
- 9qY. R. Zheng, H. B. Yang, K. Ghosh, L. Zhao, P. J. Stang, Chem. Eur. J. 2009, 15, 7203–7214;
- 9rB. H. Northrop, Y. R. Zheng, K. W. Chi, P. J. Stang, Acc. Chem. Res. 2009, 42, 1554–1563; for a representative example of calixarene-based self-sorting system, see:
- 9sY. Rudzevich, V. Rudzevich, F. Klautzsch, C. A. Schalley, V. Böhmer, Angew. Chem. 2009, 121, 3925–3929.
10.1002/ange.200805754 Google Scholar
- 10W. Jiang, H. D. F. Winkler, C. A. Schalley, J. Am. Chem. Soc. 2008, 130, 13852–13853.
- 11The pseudorotaxane structural motif is prominent among self-sorting systems, in particular with crown ethers and cucurbiturils as the wheels; for recent reports, see:
- 11aW. Jiang, D. Sattler, K. Rissanen, C. A. Schalley, Org. Lett. 2011, 13, 4502–4505;
- 11bP.-N. Chen, C.-C. Lai, S.-H. Chiu, Org. Lett. 2011, 13, 4660–4663;
- 11cW. Jiang, Q. Wang, I. Linder, F. Klautzsch, C. A. Schalley, Chem. Eur. J. 2011, 17, 2344–2348;
- 11dW. Jiang, A. Schäfer, P. C. Mohr, C. A. Schalley, J. Am. Chem. Soc. 2010, 132, 2309–2320, and Ref. [9d].
- 12For the sequence-specific self-sorting of a hetero[4]pseudorotaxane, see: G. Celtek, M. Artar, O. A. Scherman, D. Tuncel, Chem. Eur. J. 2009, 15, 10360–10363.
- 13C. Talotta, C. Gaeta, T. Pierro, P. Neri, Org. Lett. 2011, 13, 2098–2101.
- 14C. D. Gutsche, Calixarenes—An Introduction in Monographs in Supramolecular Chemistry (Ed.: ), Royal Society of Chemistry, Cambridge, 2008.
- 15For other examples of threaded architectures based on the calixarene/dialkylammonium motif, see:
- 15aC. Gaeta, C. Talotta, S. Mirra, L. Margarucci, A. Casapullo, P. Neri, Org. Lett. 2013, 15, 116–119;
- 15bC. Talotta, C. Gaeta, P. Neri, Org. Lett. 2012, 14, 3104–3107;
- 15cT. Pierro, C. Gaeta, C. Talotta, A. Casapullo, P. Neri, Org. Lett. 2011, 13, 2650–2653;
- 15dC. Gaeta, F. Troisi, P. Neri, Org. Lett. 2010, 12, 2092–2095;
- 15eG. Gattuso, A. Notti, M. F. Parisi, I. Pisagatti, M. E. Amato, A. Pappalardo, S. Pappalardo, Chem. Eur. J. 2010, 16, 2381–2385.
- 16
- 16aC. Gaeta, C. Talotta, F. Farina, M. Camalli, G. Campi, P. Neri, Chem. Eur. J. 2012, 18, 1219–1230;
- 16bC. Gaeta, C. Talotta, F. Farina, F. A. Teixeira, P. M. Marcos, J. R. Ascenso, P. Neri, J. Org. Chem. 2012, 77, 10285–10293.
- 17
- 17aN. A. Yakelis, R. G. Bergman, Organometallics 2005, 24, 3579–3581;
- 17bS. H. Strauss, Chem. Rev. 1993, 93, 927–942;
- 17cH. Nishida, N. Takada, M. Yoshimura, T. Sonoda, H. Kobayashi, Bull. Chem. Soc. Jpn. 1984, 57, 2600–2604.
- 18The preference for endo-alkyl over endo-benzyl complexation by 1H wheels was recently confirmed[15a] by DFT calculations at the B3LYP/6-31G* level of theory, which indicated that the endo-alkyl stereoisomer was more stable than the endo-benzyl stereoisomer by 3.5 kcal mol−1.
- 19See the Supporting Information for further details.
- 20The presence of 1H⋅22+⋅1tBu heteropseudo[3]rotaxanes was excluded by means of analysis of the COSY-45 spectrum of the 1:2:2 mixture of 22+, 1H, and 1tBu (see Figure S26 in the Supporting Information). In particular, analysis of the upfield negative region of the COSY-45 spectrum revealed the presence of only one aliphatic chain (α–ε in Figure 3 and Figure S26) inside a calix cavity. These data, coupled with the signal integration, are in accordance with the presence of two equivalent calix wheels at the extremities of 22+. The presence of only one type of calix wheel was confirmed by the observation of a single AX system for calixarene ArCH2Ar groups in the 3–5 ppm region of the COSY-45 spectrum.[19] In fact, as detailed previously,[13, 15] these ArCH2Ar hydrogen atoms appear as a broad singlet for the conformationally mobile free wheel, whereas they give rise to two doublets (AX system) when the wheel is conformationally blocked by pseudorotaxane formation.
- 21The slower kinetics of threading/dethreading of a tert-butylated calix[6]arene with respect to a non-tert-butylated calix[6]arene was observed previously in equilibration experiments in CDCl3.[15a]
- 22As suggested by one referee, an additional rule can be obtained from the 1:1:2 competition experiment with the two axles 22+ and 32+ and wheel 1H. The corresponding 1H NMR spectrum (see Figure S36) clearly shows the formation of (H,H)-1H⋅22+⋅1H and thus indicates a clear preference for inner- over outer-alkyl complexation. Since this additional rule is not relevant to the development of integrative self-sorting system, it was not included in the main text.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.