Direct Benzylic CH Activation for CO Bond Formation by Photoredox Catalysis†
Corresponding Author
Ganesh Pandey
Molecular Synthesis Laboratory, Centre of Biomedical Research (CBMR), Sanjay Gandhi Postgraduate Institute of Medical Science Campus, Raebareli Road, Lucknow-226014 (India)
Molecular Synthesis Laboratory, Centre of Biomedical Research (CBMR), Sanjay Gandhi Postgraduate Institute of Medical Science Campus, Raebareli Road, Lucknow-226014 (India)Search for more papers by this authorSujit Pal
Molecular Synthesis Laboratory, Centre of Biomedical Research (CBMR), Sanjay Gandhi Postgraduate Institute of Medical Science Campus, Raebareli Road, Lucknow-226014 (India)
Search for more papers by this authorRamkrishna Laha
Molecular Synthesis Laboratory, Centre of Biomedical Research (CBMR), Sanjay Gandhi Postgraduate Institute of Medical Science Campus, Raebareli Road, Lucknow-226014 (India)
Search for more papers by this authorCorresponding Author
Ganesh Pandey
Molecular Synthesis Laboratory, Centre of Biomedical Research (CBMR), Sanjay Gandhi Postgraduate Institute of Medical Science Campus, Raebareli Road, Lucknow-226014 (India)
Molecular Synthesis Laboratory, Centre of Biomedical Research (CBMR), Sanjay Gandhi Postgraduate Institute of Medical Science Campus, Raebareli Road, Lucknow-226014 (India)Search for more papers by this authorSujit Pal
Molecular Synthesis Laboratory, Centre of Biomedical Research (CBMR), Sanjay Gandhi Postgraduate Institute of Medical Science Campus, Raebareli Road, Lucknow-226014 (India)
Search for more papers by this authorRamkrishna Laha
Molecular Synthesis Laboratory, Centre of Biomedical Research (CBMR), Sanjay Gandhi Postgraduate Institute of Medical Science Campus, Raebareli Road, Lucknow-226014 (India)
Search for more papers by this authorPart of this work was carried out at the National Chemical Laboratory, Pune-411008, India. We thank the CSIR, New Delhi, India for Research Fellowships to S.P. and R.L. Financial support by the DST New Delhi through the J. C. Bose Fellowship is acknowledged. We also thank Nishamol Kuriakose (NCL, Pune) for theoretical calculations.
Graphical Abstract
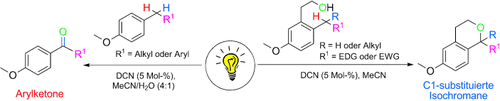
Unter Bestrahlung: 1,4-Dicyannaphthalin (DCN) und Licht aktivieren benzylische C-H-Bindungen direkt für intra- und intermolekulare C-O-Verknüpfungen. Alkylarene wurden auch direkt in Arylketone überführt, wobei Wasser als Sauerstoffquelle wirkte. EDG=elektronenschiebende Gruppe, EWG=elektronenziehende Gruppe.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201210333_sm_miscellaneous_information.pdf1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. G. Bergman, Nature 2007, 446, 391–393;
- 1bK. Godula, D. Sames, Science 2006, 312, 67–72;
- 1cJ. A. Labinger, J. E. Bercaw, Nature 2002, 417, 507–514.
- 2
- 2aN. Kuhl, M. N. Hopkinson, J. Wencel-Delord, F. Glorius, Angew. Chem. 2012, 124, 10382–10401;
10.1002/ange.201203269 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10236–10254;
- 2bL. Ackermann, Chem. Rev. 2011, 111, 1315–1345;
- 2cO. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074–1086.
- 3
- 3aO. Baudoin, Chem. Soc. Rev. 2011, 40, 4902–4911;
- 3bS.-Y. Zhang, F.-M. Zhang, Y.-Q. Tu, Chem. Soc. Rev. 2011, 40, 1937–1949.
- 4
- 4aY. Xie, Y. Yang, L. Huang, X. Zhang, Y. Zhang, Org. Lett. 2012, 14, 1238–1241;
- 4bN. Dastbaravardeh, Org. Lett. 2012, 14, 1930–1933;
- 4cY. Li, Z. Li, T. Xiong, Q. Zhang, X. Zhang, Org. Lett. 2012, 14, 3522–3525;
- 4dT. Nanjo, C. Tsukano, Y. Takemoto, Org. Lett. 2012, 14, 4270–4273;
- 4eP. Novák, A. Correa, J. Gallardo-Donaire, R. Martin, Angew. Chem. 2011, 123, 12444; Angew. Chem. Int. Ed. 2011, 50, 12236.
- 5
- 5aC. J. Li, Acc. Chem. Res. 2009, 42, 335–344;
- 5bP. Xie, Y. Xie, B. Qian, H. Zhou, C. Xia, H. Huang, J. Am. Chem. Soc. 2012, 134, 9902–9905;
- 5cZ. Li, Li. Cao, C.-J. Li, Angew. Chem. 2007, 119, 6625–6627; Angew. Chem. Int. Ed. 2007, 46, 6505–6507;
- 5dZ. Li, C. J. Li, J. Am. Chem. Soc. 2005, 127, 3672–3673.
- 6
- 6aS. J. Park, J. R. Price, M. H. Todd, J. Org. Chem. 2012, 77, 949–955;
- 6bL. Liu, P. E. Floreancig, Angew. Chem. 2010, 122, 6030–6033; Angew. Chem. Int. Ed. 2010, 49, 5894–5897;
- 6cB. Yu, T. Jiang, Y. Su, X. Pan, X. She, Org. Lett. 2009, 11, 3442–3445;
- 6dY. Zhang, C. J. Li, J. Am. Chem. Soc. 2006, 128, 4242–4243.
- 7
- 7aB. Zhang, S. K. Xiang, L. H. Zhang, Y. Cui, N. Jiao, Org. Lett. 2011, 13, 5212–5215;
- 7bÁ. Pintér, A. Sud, D. Sureshkumar, M. Klussmann, Angew. Chem. 2010, 122, 5124–5128;
10.1002/ange.201000711 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5004–5007.
- 8
- 8aG. Pandey, A. Murugan, M. Balakrishnan, Chem. Commun. 2002, 624–625;
- 8bG. Pandey, M. Karthikeyan, A. Murugan, J. Org. Chem. 1998, 63, 2867–2872;
- 8cG. Pandey, A. Krishna, K. Girija, M. Karthikeyan, Tetrahedron Lett. 1993, 34, 6631–6634;
- 8dG. Pandey, M. Sridhar, U. T. Bhalerao, Tetrahedron Lett. 1990, 31, 5373–5376;
- 8eG. Pandey, J. Org. Chem. 1988, 53, 2364–2365.
- 9G. Pandey, A. Krishna, U. T. Bhalerao, Tetrahedron Lett. 1989, 30, 1867–1870.
- 10
- 10aG. Pandey, Synlett 1992, 546–552;
- 10bG. Pandey, Top. Curr. Chem. 1993, 168, 175–221.
- 11“Photoinduced SET initiated reactions: Generation and synthetic application of arene radical cations”:
- 11aA. Krishna, Ph.D. Thesis entitled, submitted to Osmania University, Hyderabad, India, 1989;
- 11bC. Pac, A. Nakasone, H. Sakurai, J. Am. Chem. Soc. 1977, 99, 5806–5808.
- 12
- 12aGaussian 98 (Revision A.9): M. J. Frisch et al., see Supporting Information;
- 12bA. E. Reed, R. B. Weinstock, F. Weinhold, J. Chem. Phys. 1985, 83, 735–746;
- 12cD. B. Axel, J. Chem. Phys. 1993, 98, 5648–5652.
- 13
- 13aM. Uyanik, H. Okamoto, T. Yasui, K. Ishihara, Science 2010, 328, 1376–1379;
- 13bM. Uyanik, T. Yasui, K. Ishihara, Bioorg. Med. Chem. Lett. 2009, 19, 3848–3851.
- 14
- 14aR. A. Sheldon, J. K. Kochi, Metal Catalyzed Oxidations of Organic Compounds, Academic Press, New York, 1981;
- 14bG. Cainelli, G. Cardillo, Chromium Oxidations in Organic Chemistry, Springer, Berlin, 1984;
10.1007/978-3-642-69362-5 Google Scholar
- 14cM. Hudlicky, Oxidion in Organic Chemistry, American Chemical Society, Washington, DC, 1990;
- 14dM. Recupero, C. Punta, Chem. Rev. 2007, 107, 3800–3842.
- 15
- 15aK. C. Nicolaou, P. S. Baran, Y-Li. Zhong, J. Am. Chem. Soc. 2001, 123, 3183–3185;
- 15bY. Xu, J. T. Hu, J. Yan, Chin. Chem. Lett. 2012, 23, 891–894.
- 16
- 16aL. Zhang, G. Y. Ang, S. Chiba, Org. Lett. 2011, 13, 1622–1625;
- 16bT. Nagano, S. Kobayashi, Chem. Lett. 2008, 37, 1042–1045;
- 16cC. S. Yi, K. H. Kwon, D. W. Lee, Org. Lett. 2009, 11, 1567–1570;
- 16dA. J. Catino, J. M. Nichols, H. Choi, S. Gottipamula, M. P. Doyle, Org. Lett. 2005, 7, 5167–5170;
- 16eY. Bonvin, E. Callens, I. Larrosa, D. A. Henderson, J. Oldham, A. J. Burton, A. G. M. Barrett, Org. Lett. 2005, 7, 4549–4552;
- 16fN. H. Lee, C. S. Lee, D. S. Jung, Tetrahedron Lett. 1998, 39, 1385–1388.
- 17
- 17aG. Yang, Q. Zhang, H. Miao, X. Tong, J. Xu, Org. Lett. 2005, 7, 263–266;
- 17bK. Ohkubo, S. Fukuzumi, Org. Lett. 2000, 2, 3647–3650;
- 17cR. Lechner, S. Kümmel, B. König, Photochem. Photobiol. Sci. 2010, 9, 1367–1377.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.