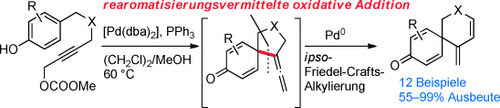
Palladium-Catalyzed Intramolecular ipso-Friedel–Crafts Alkylation of Phenols and Indoles: Rearomatization-Assisted Oxidative Addition†
Dr. Tetsuhiro Nemoto
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Search for more papers by this authorZengduo Zhao
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Search for more papers by this authorTakuya Yokosaka
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Search for more papers by this authorYuta Suzuki
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Search for more papers by this authorRiliga Wu
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yasumasa Hamada
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)===Search for more papers by this authorDr. Tetsuhiro Nemoto
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Search for more papers by this authorZengduo Zhao
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Search for more papers by this authorTakuya Yokosaka
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Search for more papers by this authorYuta Suzuki
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Search for more papers by this authorRiliga Wu
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yasumasa Hamada
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)
Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675 (Japan)===Search for more papers by this authorThis work was supported by Grant-in Aid for Scientific Resarch from the JSPS (Japan), the Research Foundation for Pharmaceutical Sciences, Chiba Univsersity, and the Inohana Foundation (Chiba University).
Graphical Abstract
Inspiroierend: Eine neuartige Synthese von Spirocyclen basierend auf einer Palladium-katalysierten intramolekularen ipso-Friedel-Crafts-Alkylierung von Phenolen (siehe Schema; dba=Dibenzylidenaceton) und Indolen wird beschrieben. Mechanistische Studien belegen, dass die Reaktion über eine präzedenzlose Rearomatisierungs-unterstützte oxidative Addition verläuft.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange_201209317_sm_miscellaneous_information.pdf2.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Representative examples, see:
- 1aR. Imbos, A. J. Minnaard, B. L. Feringa, J. Am. Chem. Soc. 2002, 124, 184;
- 1bS. Canesi, D. Bouchu, M. A. Ciufolini, Angew. Chem. 2004, 116, 4436;
10.1002/ange.200460178 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4336;
- 1cY. Hayashi, H. Gotoh, T. Tamura, H. Yamagichi, R. Masui, M. Shoji, J. Am. Chem. Soc. 2005, 127, 16028;
- 1dQ. Liu, T. Rovis, J. Am. Chem. Soc. 2006, 128, 2552;
- 1eN. T. Vo, R. D. Pace, F. O’Hara, M. J. Gaunt, J. Am. Chem. Soc. 2008, 130, 404;
- 1fB. A. Mendelsohn, M. A. Ciufolini, Org. Lett. 2009, 11, 4736;
- 1gH. Liang, M. A. Ciufolini, Org. Lett. 2010, 12, 1760;
- 1hQ. Gu, S.-L. You, Org. Lett. 2011, 13, 5192.
- 2For reviews, see:
- 2aS. T. Roche, J. A. Porco, Jr., Angew. Chem. 2011, 123, 4154;
10.1002/ange.201006017 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4068;
- 2bC.-X. Zhuo, W. Zhang, S.-L. You, Angew. Chem. 2012, 124, 12834; Angew. Chem. Int. Ed. 2012, 51, 12662.
- 3
- 3aT. Nemoto, Y. Ishige, M. Yoshida, Y. Kohno, M. Kanematsu, Y. Hamada, Org. Lett. 2010, 12, 5020;
- 3bQ.-F. Wu, W.-B. Liu, C.-X. Zhuo, Z.-Q. Rong, K.-Y. Ye, S.-L. You, Angew. Chem. 2011, 123, 4547; Angew. Chem. Int. Ed. 2011, 50, 4455;
- 3cS. Rousseaux, J. García-Fortanet, M. A. Del Aguila Sanchez, S. L. Buchwald, J. Am. Chem. Soc. 2011, 133, 9282;
- 3dM. Yoshida, T. Nemoto, Z. Zhao, Y. Ishige, Y. Hamada, Tetrahedron: Asymmetry 2012, 23, 859.
- 4Other representative examples of the synthesis of spirocyclohexadienones. Halocyclization:
- 4aX. Zhang, R. C. Larock, J. Am. Chem. Soc. 2005, 127, 12230;
- 4bB.-X. Tang, D.-J. Tang, S. Tang, Q.-F. Yu, Y.-H. Zhang, Y. Liang, P. Zhong, J.-H. Li, Org. Lett. 2008, 10, 1063;
- 4cQ. Yin, S.-L. You, Org. Lett. 2012, 14, 3526; Radical cyclization:
- 4dF. González-López de Turiso, D. P. Curran, Org. Lett. 2005, 7, 151;
- 4eT. Lanza, R. Leardini, M. Minozzi, D. Nanni, P. Spagnolo, G. Zanardi, Angew. Chem. 2008, 120, 9581;
10.1002/ange.200804333 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9439; Hypervalent iodine reagents:
- 4fT. Dohi, A. Maruyama, M. Yoshimura, K. Morimoto, H. Tohma, Y. Kita, Angew. Chem. 2005, 117, 6349;
10.1002/ange.200501688 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6193;
- 4gY. Dohi, Y. Minamitsuji, A. Maruyama, S. Hirose, Y. Kita, Org. Lett. 2008, 10, 3559; Review;
- 4hV. V. Zhdankin, P. J. Stang, Chem. Rev. 2008, 108, 5299; Other metal-mediated methods:
- 4iF. C. Pigge, J. J. Coniglio, R. Dalvi, J. Am. Chem. Soc. 2006, 128, 3498;
- 4jS. Chiba, L. Zhang, J.-Y. Lee, J. Am. Chem. Soc. 2010, 132, 7266.
- 5K. Tsutsumi, S. Ogoshi, S. Nishiguchi, H. Kurosawa, J. Am. Chem. Soc. 1998, 120, 1938.
- 6For reviews, see:
- 6aJ. Tsuji, Palladium Reagents and Catalysts: New Perspectives for the 21st Century, Wiley, Chichester, 2004, Chapter 6;
10.1002/0470021209 Google Scholar
- 6bH. Ohno, Chem. Pharm. Bull. 2005, 53, 1211;
- 6cL.-N. Guo, X.-H. Duan, Y.-M. Liang, Acc. Chem. Res. 2011, 44, 111;
- 6dM. Yoshida, Chem. Pharm. Bull. 2012, 60, 285.
- 7For examples of the synthesis of aza-spirocycles using a transition-metal-catalyzed dearomatization of indoles, see:
- 7aQ.-F. Wu, H. He, W.-B. Liu, S.-L. You, J. Am. Chem. Soc. 2010, 132, 11418;
- 7bY. Liu, W. Xu, X. Wang, Org. Lett. 2010, 12, 1448;
- 7cG. Cera, P. Crispino, M. Monari, M. Bandini, Chem. Commun. 2011, 47, 7803;
- 7dG. Cera, M. Chiarucci, A. Mazzanti, M. Mancinelli, M. Bandini, Org. Lett. 2012, 14, 1350;
- 7eQ.-F. Wu, C. Zheng, S.-L. You, Angew. Chem. 2012, 124, 1712; Angew. Chem. Int. Ed. 2012, 51, 1680;
- 7fK.-J. Wu, L.-X. Dai, S.-L. You, Org. Lett. 2012, 14, 3772.
- 8For detailed data, see the Supporting Information.
- 9No reaction occurred when a propargyl alcohol derivative was treated under the same reaction conditions. The reaction was very sluggish when using a propargyl acetate derivative as the substrate.
- 10It is proposed that η3-propargylpalladium(II) species react with nucleophiles on the central carbon atom to produce metallacyclobutene intermediates such as E. For references, see:
- 10aC. P. Casey, J. R. Nash, C. S. Yi, A. D. Selmeczy, S. Chung, D. R. Powell, R. K. Hayashi, J. Am. Chem. Soc. 1998, 120, 722;
- 10bM. Yoshida, M. Fujita, M. Ihara, Org. Lett. 2003, 5, 3325;
- 10cH. Ohno, H. Hamaguchi, M. Ohata, S. Kosaka, T. Tanaka, J. Am. Chem. Soc. 2004, 126, 8744.
- 11H. David, L. Dupuis, M.-G. Guillerez, F. Guibé, Tetrahedron Lett. 2000, 41, 3335–3338.
- 12For an oxidative addition of C
 Namide bonds in spirocyclohexadienones to transition metals, see: N. A. Braun, M. A. Ciufolini, K. Peters, E.-M. Peters, Tetrahedron Lett. 1998, 39, 4667.
Namide bonds in spirocyclohexadienones to transition metals, see: N. A. Braun, M. A. Ciufolini, K. Peters, E.-M. Peters, Tetrahedron Lett. 1998, 39, 4667.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.