Strong Electronic and Counterion Effects on Geminal Digold Formation and Reactivity as Revealed by Gold(I)–Aryl Model Complexes†
Dieter Weber
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516-3290 (USA)
Search for more papers by this authorT. David Jones
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516-3290 (USA)
Search for more papers by this authorLaura L. Adduci
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516-3290 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Michel R. Gagné
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516-3290 (USA)
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516-3290 (USA)Search for more papers by this authorDieter Weber
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516-3290 (USA)
Search for more papers by this authorT. David Jones
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516-3290 (USA)
Search for more papers by this authorLaura L. Adduci
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516-3290 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Michel R. Gagné
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516-3290 (USA)
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516-3290 (USA)Search for more papers by this authorWe thank Dr. Peter White and LLA for assistance with crystallography ([email protected] and [email protected] for correspondence). The Fulbright Foreign Student Fulbright Program (for D.W.) is gratefully acknowledged as is the National Institute for General Medical Sciences (GM-60578).
Graphical Abstract
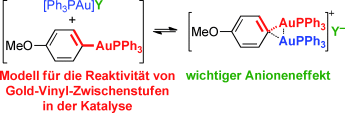
Die geminale Diaurierung von [Ph3PAu(Aryl)]-Komplexen wurde als Modell untersucht, um Einblick in die intermediäre Bildung von geminalen diaurierten Gold(I)-Vinylkomplexen in der Katalyse zu gewinnen (siehe Schema). Die Ergebnisse tragen zum Verständnis der Faktoren bei, die die Stabilität, Reaktivität und Dynamik dieser metallorganischen Zwischenstufen bestimmen.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201107659_sm_miscellaneous_information.pdf868.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aN. Krause, C. Winter, Chem. Rev. 2011, 111, 1994–2009;
- 1bA. Corma, A. Leyva-Pérez, M. J. Sabater, Chem. Rev. 2011, 111, 1657–1712;
- 1cT. C. Boorman, I. Larrosa, Chem. Soc. Rev. 2011, 40, 1910–1925;
- 1dC. Aubert, L. Fensterbank, P. Garcia, M. Malacria, A. Simonneau, Chem. Rev. 2011, 111, 1954–1993;
- 1eM. Bandini, Chem. Soc. Rev. 2011, 40, 1358–1367;
- 1fA. Pradal, P. Y. Toullec, V. Michelet, Synthesis 2011, 1501–1514;
- 1gM. Rudolph, A. S. K. Hashmi, Chem. Commun. 2011, 47, 6536–6544;
- 1hS. Sengupta, X. Shi, ChemCatChem 2010, 2, 609–619;
- 1iA. Fürstner, Chem. Soc. Rev. 2009, 38, 3208–3221;
- 1jZ. Li, C. Brouwer, C. He, Chem. Rev. 2008, 108, 3239–3265;
- 1kR. A. Widenhoefer, Chem. Eur. J. 2008, 14, 5382–5391;
- 1lA. S. K. Hashmi, M. Rudolph, Chem. Soc. Rev. 2008, 37, 1766–1775;
- 1mD. J. Gorin, B. D. Sherry, F. D. Toste, Chem. Rev. 2008, 108, 3351–3378;
- 1nE. Jiménez-Núñez, A. M. Echavarren, Chem. Rev. 2008, 108, 3326–3350;
- 1oA. Arcadi, Chem. Rev. 2008, 108, 3266–3325;
- 1pA. S. K. Hashmi, Chem. Rev. 2007, 107, 3180–3211;
- 1qD. J. Gorin, F. D. Toste, Nature 2007, 446, 395–403;
- 1rA. S. K. Hashmi, G. J. Hutchings, Angew. Chem. 2006, 118, 8064–8105;
10.1002/ange.200602454 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7896–7936;
- 1sA. Fürstner, P. W. Davies, Angew. Chem. 2007, 119, 3478–3519;
10.1002/ange.200604335 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3410–3449.
- 2The isolation of gold intermediates and model complexes has been reviewed:
- 2aA. S. K. Hashmi, Angew. Chem. 2010, 122, 5360–5369;
10.1002/ange.200907078 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5232–5241;
- 2bH. G. Raubenheimer, H. Schmidbaur, S. Afr. J. Sci. 2011, 107( 3–4), 1–13;
- 2cA. S. K. Hashmi, Gold Bull. 2009, 42, 275–279;
- 2dH. Schmidbaur, A. Schier, Organometallics 2010, 29, 2–23.
- 3Most often E+ is a proton (see ref. [25]), but halonium ions and metal electrophiles were also used, see:
- 3aH. A. Wegner, M. Auzias, Angew. Chem. 2011, 123, 8386–8397;
10.1002/ange.201101603 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8236–8247;
- 3bJ. J. Hirner, Y. Shi, S. A. Blum, Acc. Chem. Res. 2011, 44, 603–613, and references therein. Model complexes were used to understand E+ reactivity better. For selected examples, see:
- 3cN. P. Mankad, F. D. Toste, J. Am. Chem. Soc. 2010, 132, 12859–12861;
- 3dA. S. K. Hashmi, T. D. Ramamurthi, F. Rominger, J. Organomet. Chem. 2009, 694, 592–597;
- 3eA. S. K. Hashmi, T. D. Ramamurthi, M. H. Todd, A. S.-K. Tsang, K. Graf, Aust. J. Chem. 2010, 63, 1619–1626;
- 3fD. Weber, M. R. Gagné, Chem. Commun. 2011, 47, 5172–5174.
- 4
- 4aD. Weber, M. A. Tarselli, M. R. Gagné, Angew. Chem. 2009, 121, 5843–5846; Angew. Chem. Int. Ed. 2009, 48, 5733–5736;
- 4bD. Weber, M. R. Gagné, Org. Lett. 2009, 11, 4962–4965.
- 5
- 5aH. Schmidbaur, A. Schier, Chem. Soc. Rev. 2008, 37, 1931–1951;
- 5bH. Schmidbaur, Gold Bull. 2000, 33, 1–10;
- 5cH. Schmidbaur, Chem. Soc. Rev. 1995, 24, 391–400.
- 6
- 6aG. Seidel, C. W. Lehmann, A. Fürstner, Angew. Chem. 2010, 122, 8644–8648;
10.1002/ange.201003349 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8466–8470;
- 6bK. A. Porter, A. Schier, H. Schmidbaur, Organometallics 2003, 22, 4922–4927;
- 6cA. N. Nesmeyanov, E. G. Perevalova, K. I. Grandberg, D. A. Lemenovskii, Izv. Akad. Nauk SSSR Ser. Khim. 1974, 5, 1124–1137;
- 6dM. Osawa, M. Hoshino, D. Hashizume, Dalton Trans. 2008, 2248–2252;
- 6eR. Usón, A. Laguna, E. J. Fernandez, A. Media, P. G. Jones, J. Organomet. Chem. 1988, 350, 129–138;
- 6fH. Schmidbaur, Y. Inoguchi, Chem. Ber. 1980, 113, 1646–1653;
- 6gM. Alcarazo, C. W. Lehmann, A. Anoop, W. Thiel, A. Fürstner, Nat. Chem. 2009, 1, 295–301;
- 6hJ. Vicente, A. R. Singhal, P. G. Jones, Organometallics 2002, 21, 5887–5900; see also ref. [10].
- 7
- 7aH. G. Raubenheimer, H. Schmidbaur, Organometallics 2011, DOI: ;
- 7bR. Hoffmann, Angew. Chem. 1982, 94, 725–739;
10.1002/ange.19820941002 Google ScholarAngew. Chem. Int. Ed. Engl. 1982, 21, 711–724.
- 8We have attempted the synthesis of non-heteroatom and non-cyclopropyl-stabilized digold vinyl complexes, but these complexes rapidly decomposed through CC bond forming homocoupling as published by Fürstner et al. (ref. [6a]). The same reactivity was also observed with aryl complexes, but at a much lower rate. Electron-deficient digold aryl complexes (e.g. 1 c) decomposed at a significantly higher rate than electron-rich digold aryl complexes (e.g. 1 a).
- 9For a complete list of synthesized mononuclear and dinuclear gold aryl complexes, see the Supporting Information.
- 10
- 10aK. I. Grandberg, V. P. Dyadchenko, J. Organomet. Chem. 1994, 474, 1–24;
- 10bK. I. Grandberg, Russ. Chem. Rev. 1982, 51, 249–262.
10.1070/RC1982v051n03ABEH002838 Google Scholar
- 11C. Croix, A. Balland-Longeau, H. Allouchi, M. Giorgi, A. Duchêne, J. Thibonnet, J. Organomet. Chem. 2005, 690, 4835–4843.
- 12CDCC 851149 ([(2,5-(OCH3)2-C6H3)AuPPh3]) and 851150 ([(3-CH3-C6H4)AuPPh3]) contain the supplementary crystallographic data. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13
- 13aN. Mézailles, L. Ricard, F. Gagosz, Org. Lett. 2005, 7, 4133–4136; for a review on silver-free gold catalysts, see:
- 13bH. Schmidbaur, A. Schier, Z. Naturforsch. B 2011, 66, 329–350.
- 14CCDC 851147 ([(2,5-(OCH3)2-C6H3)(AuPPh3)2]NTf2) and 851148 ([(2-CH3-C6H4)(AuPPh3)2]NTf2) contain the supplementary crystallographic data. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15A. N. Nesmeyanov, E. G. Perevalova, K. I. Grandberg, D. A. Lemenovskii, T. V. Baukova, O. B. Afanassova, J. Organomet. Chem. 1974, 65, 131–144.
- 16The formation of a trigold species was previously reported: R. D. Rakhimov, K. P. Butin, K. I. Grandberg, J. Organomet. Chem. 1994, 464, 253–260.
- 17See the Supporting Information for details.
- 18Concentration of both reagents was 0.004 M; see the Supporting Information for details.
- 19Protodemetalation of 1 c could not be performed due to overlapping aryl and PPh3 signals in the 1H NMR spectrum.
- 20A slight downfield shift indicating digold formation was also observed during the protodemetalation of 1 b, but was less pronounced than with 1 a.
- 21See the Supporting Information for reaction conditions and tables.
- 22An activation of a Brønsted acid by a gold Lewis acid was recently reported: O. Kanno, W. Kuriyama, Z. J. Wang, F. D. Toste, Angew. Chem. 2011, 123, 10093–10096; Angew. Chem. Int. Ed. 2011, 50, 9919–9922.
- 23J. H. Koh, A. O. Larsen, M. R. Gagné, Org. Lett. 2001, 3, 1233–1236.
- 24See the Supporting Information for details.
- 25Blum has demonstrated that the Hammond vinyl was not kinetically basic in comparison to [Ph3PAuPh], see:
- 25aK. E. Roth, S. A. Blum, Organometallics 2010, 29, 1712–1716;
- 25bL.-P. Liu, B. Xu, M. S. Mashuta, G. B. Hammond, J. Am. Chem. Soc. 2008, 130, 17642–17643; in the reaction of gold complexes with protons H2O molecules play a crucial role as proton shuttles, see:
- 25cC. M. Krauter, A. S. K. Hashmi, M. Pernpointner, ChemCatChem 2010, 2, 1226–1230.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.