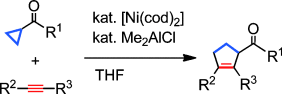
[3+2] Cycloaddition Reaction of Cyclopropyl Ketones with Alkynes Catalyzed by Nickel/Dimethylaluminum Chloride†
Takashi Tamaki
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan)
Search for more papers by this authorDr. Masato Ohashi
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan)
Center for Atomic and Molecular Technologies, Osaka University, Suita, Osaka 565-0871 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Sensuke Ogoshi
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan)
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan)Search for more papers by this authorTakashi Tamaki
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan)
Search for more papers by this authorDr. Masato Ohashi
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan)
Center for Atomic and Molecular Technologies, Osaka University, Suita, Osaka 565-0871 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Sensuke Ogoshi
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan)
Department of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871 (Japan)Search for more papers by this authorThis work was supported by the Grants-in-Aid for Scientific Research (No. 21245028) and “Molecular Activation Directed toward Straightforward Synthesis (No. 23105546) from MEXT. T.T. expresses his special thanks for the research fellowships for young scientists from the Japan Society for the Promotion of Science (JSPS) and the Global COE Program ”Global Education and Research Center for Bio-Environmental Chemistry“ of Osaka University.
Graphical Abstract
Al aktiviert, Cl stabilisiert: In der Titelreaktion aktiviert das Organoaluminiumreagens die Carbonylgruppe des Cyclopropylketons, indem es über das Aluminiumatom an deren Sauerstoffatom koordiniert. Außerdem stabilisiert es eine Zwischenstufe der Reaktion durch Koordination von Chlorid an das Nickelzentrum. cod=1,5-Cyclooctadien, THF=Tetrahydrofuran.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201106174_sm_miscellaneous_information.pdf2.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For review to see: M. Rubin, M. Rubina, V. Gevorgyan, Chem. Rev. 2007, 107, 3117–3179.
- 2
- 2aS. Saito, M. Masuda, S. Komagawa, J. Am. Chem. Soc. 2004, 126, 10540–10541;
- 2bS. Komagawa, S. Saito, Angew. Chem. 2006, 118, 2506–2509;
10.1002/ange.200504050 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2446–2449;
- 2cS. Saito, S. Komagawa, I. Azumaya, M. Masuda, J. Org. Chem. 2007, 72, 9114–9120;
- 2dK. Maeda, S. Saito, Tetrahedron Lett. 2007, 48, 3173–3176.
- 3
- 3aP. A. Wender, H. Rieck, M. Fuji, J. Am. Chem. Soc. 1998, 120, 10976–10977;
- 3bP. A. Wender, A. J. Dyckman, C. O. Husfeld, M. J. C. Scanio, Org. Lett. 2000, 2, 1609–1611;
- 3cP. A. Wender, C. M. Barzilay, A. J. Dyckman, J. Am. Chem. Soc. 2001, 123, 179–180.
- 4P. A. Wender, T. M. Pedersen, M. J. C. Scanio, J. Am. Chem. Soc. 2002, 124, 15154–15155.
- 5
- 5aP. Binger, Q.-H. Lü, P. Wedemann, Angew. Chem. 1985, 97, 333–334; Angew. Chem. Int. Ed. Engl. 1985, 24, 316–317; Intramolecular cycloaddition of methylenecyclopropanes with alkynes:
- 5bS. A. Bapuji, W. B. Motherwell, M. Shipman, Tetrahedron Lett. 1989, 30, 7107–7110;
- 5cH. Corlay, R. T. Lewis, W. B. Motherwell, M. Shipman, Tetrahedron 1995, 51, 3303–3318;
- 5dM. Lautens, Y. Ren, P. H. M. Delanghe, J. Am. Chem. Soc. 1994, 116, 8821–8822;
- 5eM. Lautens, Y. Ren, J. Am. Chem. Soc. 1996, 118, 9597–9605;
- 5fA. Delgado, J. R. Rodríguez, L. Castedo, J. L. Mascareñas, J. Am. Chem. Soc. 2003, 125, 9282–9283;
- 5gR. García-Fandiño, M. Gulías, L. Castedo, J. R. Granja, J. L. Mascareñas, D. J. Cárdenas, Chem. Eur. J. 2008, 14, 272–281;
- 5hB. Yao, Y. Li, Z. Liang, Y. Zhang, Org. Lett. 2011, 13, 640–643; Nickel-catalyzed isomerization of vinylcyclopropane:
- 5iM. Suginome, T. Matsuda, T. Yoshimoto, Y. Ito, Organometallics 2002, 21, 1537–1539;
- 5jG. Zuo, J. Louie, Angew. Chem. 2004, 116, 2327–2329; Angew. Chem. Int. Ed. 2004, 43, 2277–2279; Intramolecular cycloaddition of vinylcyclopropanes with alkynes:
- 5kL. Jiao, M. Lin, Z.-X. Yu, Chem. Commun. 2010, 46, 1059–1061.
- 6
- 6aS. Ogoshi, M. Nagata, H. Kurosawa, J. Am. Chem. Soc. 2006, 128, 5350–5351;
- 6bL. Liu, J. Montgomery, J. Am. Chem. Soc. 2006, 128, 5348–5349;
- 6cG. C. Lloyd-Jones, Angew. Chem. 2006, 118, 6942–6944;
10.1002/ange.200602629 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6788–6790.
- 7T. Tamaki, M. Nagata, M. Ohashi, S. Ogoshi, Chem. Eur. J. 2009, 15, 10083–10091.
- 8T. Ichiyanagi, S. Kuniyama, M. Shimizu, T. Fujisawa, Chem. Lett. 1997, 1149–1150.
- 9TiCl4 was known as an effective Lewis acid to mediate the [3+2] cycloaddition of cyclopropyl ketones with alkynes: V. K. Yadav, V. Sriramurthy, Angew. Chem. 2004, 116, 2723–2725; Angew. Chem. Int. Ed. 2004, 43, 2669–2671.
- 10H.-U. Gonzenbach, K. Schaffner, B. Blank, H. Fischer, Helv. Chim. Acta 1973, 56, 1741–1752.
- 11See the Supporting Information for details.
- 12The bulkiness of triisopropylsilylacetylene retards its homodimerization:
- 12aN. Matsuyama, H. Tsurugi, T. Satoh, M. Miura, Adv. Synth. Catal. 2008, 350, 2274–2278;
- 12bN. Matsuyama, K. Hirano, T. Satoh, M. Miura, J. Org. Chem. 2009, 74, 3576–3578;
- 12cM. Shirakura, M. Suginome, Org. Lett. 2009, 11, 523–526.
- 13
- 13aD. R. Fahey, J. E. Mahan, J. Am. Chem. Soc. 1977, 99, 2501–2508;
- 13bL. Cronin, C. L. Higgitt, R. Karch, R. N. Perutz, Organometallics 1997, 16, 4920–4928;
- 13cS. A. Johnson, C. W. Huff, F. Mustafa, M. Saliba, J. Am. Chem. Soc. 2008, 130, 17278–17280.
- 14
- 14aD. A. Vicic, W. D. Jones, J. Am. Chem. Soc. 1999, 121, 7606–7617;
- 14bM. Hernández, G. Miralrio, A. Avévalo, S. Bernès, J. J. García, C. López, P. M. Maitlis, F. del Rio, Organometallics 2001, 20, 4061–4071.
- 15Although an η2-ketone complex of 1 a was formed in the reaction of 1 a with [Ni(cod)2] and PCy3 quantitatively, 1 h gave the corresponding η2-ketone complex only in 15 % yield under the same reaction conditions.
- 16The cyclopropyl ketone 1 n was employed in Ref. [17] and did not undergo intramolecular [3+2] cycloaddition reaction. See the Supporting Information for details.

- 17Z. Lu, M. Shen, T. P. Yoon, J. Am. Chem. Soc. 2011, 133, 1162–1164.
- 18
- 18aS. Ogoshi, T. Yoshida, T. Nishida, M. Morita, H. Kurosawa, J. Am. Chem. Soc. 2001, 123, 1944–1950;
- 18bS. Ogoshi, H. Kamada, H. Kurosawa, Tetrahedron 2006, 62, 7583–7588.
- 19
- 19aK. K. D. Amarasinghe, S. K. Chowdhury, M. J. Heeg, J. Montgomery, Organometallics 2001, 20, 370–372;
- 19bH. P. Hratchian, S. K. Chowdhury, V. M. Gutiérrez-García, K. K. D. Amarasinghe, M. J. Heeg, H. B. Schlegel, J. Montgomery, Organometallics 2004, 23, 4636–4646;
- 19cJ. Cámpora, C. M. Maya, P. Palma, E. Carmona, C. Graiff, A. Tiripicchio, Chem. Commun. 2003, 1742–1743.
- 20The treatment of nickel compounds with carbon monoxide can yield [Ni(CO)4] (extremely toxic). The reaction mixture must be handled in a well-ventilated fume hood.
- 21η2-Coordination of carbonyl group to nickel(0) enhanced by Me2AlOTf has been reported: M. Ohashi, H. Saijo, T. Arai, S. Ogoshi, Organometallics 2010, 29, 6534–6540.
- 22S. Ogoshi, M. Ueta, T. Arai, H. Kurosawa, J. Am. Chem. Soc. 2005, 127, 12810–12811.
- 23M. Ohashi, M. Ikawa, S. Ogoshi, Organometallics 2011, 30, 2765–2774.
- 24S. Ogoshi, A. Nishimura, M. Ohashi, Org. Lett. 2010, 12, 3450–3452.
- 25CCDC 841838 (3 ag) and 841839 (6) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.