Scandium-Catalyzed Silylation of Aromatic CH Bonds†
Dr. Juzo Oyamada
Organometallic Chemistry Laboratory and Advanced Catalyst Research Team, RIKEN Advanced Science Institute, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)
Search for more papers by this authorDr. Masayoshi Nishiura
Organometallic Chemistry Laboratory and Advanced Catalyst Research Team, RIKEN Advanced Science Institute, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhaomin Hou
Organometallic Chemistry Laboratory and Advanced Catalyst Research Team, RIKEN Advanced Science Institute, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)
Organometallic Chemistry Laboratory and Advanced Catalyst Research Team, RIKEN Advanced Science Institute, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)Search for more papers by this authorDr. Juzo Oyamada
Organometallic Chemistry Laboratory and Advanced Catalyst Research Team, RIKEN Advanced Science Institute, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)
Search for more papers by this authorDr. Masayoshi Nishiura
Organometallic Chemistry Laboratory and Advanced Catalyst Research Team, RIKEN Advanced Science Institute, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhaomin Hou
Organometallic Chemistry Laboratory and Advanced Catalyst Research Team, RIKEN Advanced Science Institute, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)
Organometallic Chemistry Laboratory and Advanced Catalyst Research Team, RIKEN Advanced Science Institute, 2-1 Hirosawa, Wako, Saitama 351-0198 (Japan)Search for more papers by this authorThis work was partly supported by a Grant-in-Aid for Scientific Research (S) from JSPS (No. 21225004).
Graphical Abstract
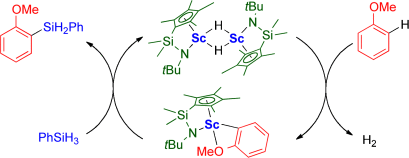
Eine regioselektive, H-Akzeptor-freie C-H-Silylierung von Anisolen und Alkoxybenzolen mit Hydrosilanen gelingt in Gegenwart eines Halbsandwich-Scandiumkatalysators. Die Reaktion verläuft über eine ortho-C-H-Aktivierung des Anisols durch eine Scandiumhydridspezies mit nachfolgender Silylierung des entstehenden Scandium-2-anisyls mit einem Hydrosilan (siehe Schema).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201105636_sm_miscellaneous_information.pdf1.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. Kakiuchi, K. Igi, M. Matsumoto, N. Chatani, S. Murai, Chem. Lett. 2001, 422;
- 1bF. Kakiuchi, K. Igi, M. Matsumoto, T. Hayamizu, N. Chatani, S. Murai, Chem. Lett. 2002, 396;
- 1cF. Kakiuchi, M. Matsumoto, K. Tsuchiya, K. Igi, T. Hayamizu, N. Chatani, S. Murai, J. Organomet. Chem. 2003, 686, 134;
- 1dH. Ihara, M. Suginome, J. Am. Chem. Soc. 2009, 131, 7502;
- 1eH. F. T. Klare, M. Oestreich, J. Ito, H. Nishiyama, Y. Ohki, K. Tatsumi, J. Am. Chem. Soc. 2011, 133, 3312.
- 2
- 2aW. A. Gustavson, P. S. Epstein, M. D. Curtis, Organometallics 1982, 1, 884;
- 2bB. Lu, J. R. Falck, Angew. Chem. 2008, 120, 7618; Angew. Chem. Int. Ed. 2008, 47, 7508;
- 2cE. M. Simmons, J. F. Hartwig, J. Am. Chem. Soc. 2010, 132, 17092.
- 3
- 3aP. I. Djurovich, A. R. Dolich, D. H. Berry, J. Chem. Soc. Chem. Commun. 1994, 1897;
- 3bK. Ezbiansky, P. I. Djurovich, M. LaForest, D. J. Sinning, R. Zayes, D. H. Berry, Organometallics 1998, 17, 1455.
- 4
- 4aY. Uchimaru, A. M. M. E. Sayed, M. Tanaka, Organometallics 1993, 12, 2065;
- 4bN. Tsukada, J. F. Hartwig, J. Am. Chem. Soc. 2005, 127, 5022;
- 4cM. Murata, N. Fukuyama, J. Wada, S. Watanabe, Y. Masuda, Chem. Lett. 2007, 36, 910.
- 5
- 5aT. Sakakura, Y. Tokunaga, T. Sodeyama, M. Tanaka, Chem. Lett. 1987, 2375;
- 5bM. Tobisu, Y. Ano, N. Chatani, Chem. Asian J. 2008, 3, 1585;
- 5cT. Ureshino, T. Yoshida, Y. Kuninobu, K. Takai, J. Am. Chem. Soc. 2010, 132, 14324.
- 6
- 6aT. Ishiyama, K. Sato, Y. Nishio, N. Miyaura, Angew. Chem. 2003, 115, 5504;
10.1002/ange.200352399 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5346;
- 6bT. Ishiyama, K. Sato, Y. Nishio, T. Saiki, N. Miyaura, Chem. Commun. 2005, 5065;
- 6cT. Saiki, Y. Nishio, T. Ishiyama, N. Miyaura, Organometallics 2006, 25, 6068.
- 7
- 7aM. Ishikawa, S. Okazaki, A. Naka, H. Sakamoto, Organometallics 1992, 11, 4135;
- 7bA. Naka, K. K. Lee, K. Yoshizawa, T. Yamabe, M. Ishikawa, Organometallics 1999, 18, 4524.
- 8
- 8aM. Ishikawa, A. Naka, J. Ohshita, Organometallics 1993, 12, 4987;
- 8bN. A. Williams, Y. Uchimaru, M. Tanaka, J. Chem. Soc. Chem. Commun. 1995, 1129;
- 8cN. A. Williams, Y. Uchimaru, M. Tanaka, Dalton Trans. 2003, 236.
- 9
- 9aK. Tamao, N. Ishida, T. Tanaka, M. Kumada, Organometallics 1983, 2, 1694;
- 9bK. Sato, M. Kira, H. Sakurai, Tetrahedron Lett. 1989, 30, 4375;
- 9cG. R. Jones, Y. Landais, Tetrahedron 1996, 52, 7599. See also Ref. [1d].
- 10aS. E. Denmark, R. F. Sweis, in Metal-Catalyzed Cross-Coupling Reactions, Vol. 1, 2nd ed. ), Wiley-VCH, Weinheim, 2004, pp. 163–216;
10.1002/9783527619535.ch4 Google Scholar
- 10bY. Hatanaka, S. Fukushima, T. Hiyama, Chem. Lett. 1989, 1711;
- 10cY. Hatanaka, T. Hiyama, Chem. Lett. 1989, 2049;
- 10dY. Hatanaka, T. Hiyama, Tetrahedron Lett. 1990, 31, 2719;
- 10eH. Matsuhashi, S. Asai, K. Hirabayashi, Y. Hatanaka, A. Mori, T. Hiyama, Bull. Chem. Soc. Jpn. 1997, 70, 1943;
- 10fK. Hirabayashi, Y. Nishihara, A. Mori, T. Hiyama, Tetrahedron Lett. 1998, 39, 7893;
- 10gS. Oi, M. Moro, Y. Inoue, Organometallics 2001, 20, 1036;
- 10hS. Oi, A. Taira, Y. Honma, Y. Inoue, Org. Lett. 2003, 5, 97.
- 11
- 11aD. Bai, S. Han, Z.-H. Lu, S. Wang, Can. J. Chem. 2008, 86, 230;
- 11bT. Kumagai, S. Itsuno, Macromolecules 2002, 35, 5323;
- 11cX.-M. Liu, C. He, J. Huang, J. Xu, Chem. Mater. 2005, 17, 434;
- 11dY. You, C.-G. An, J.-J. Kim, S. Y. Park, J. Org. Chem. 2007, 72, 6241;
- 11eA. Iida, K. Nagura, S. Yamaguchi, Chem. Asian J. 2008, 3, 1456.
- 12For examples of CH bond activation by rare-earth alkyl complexes, see:
- 12aP. L. Watson, J. Chem. Soc. Chem. Commun. 1983, 276;
- 12bP. L. Watson, J. Am. Chem. Soc. 1983, 105, 6491;
- 12cP. L. Watson, Acc. Chem. Res. 1985, 18, 51;
- 12dM. E. Thompson, S. M. Baxter, A. R. Bulls, B. J. Burger, M. C. Nolan, B. D. Santarsiero, W. P. Schaefer, J. E. Bercaw, J. Am. Chem. Soc. 1987, 109, 203;
- 12eK. H. den Haan, Y. Wielstra, J. H. Teuben, Organometallics 1987, 6, 2053;
- 12fW. J. Evans, L. R. Chamberlain, T. A. Ulibarri, J. W. Ziller, J. Am. Chem. Soc. 1988, 110, 6423;
- 12gW. J. Evans, T. A. Ulibarri, J. W. Ziller, Organometallics 1991, 10, 134;
- 12hM. Booij, A. Meetsma, J. H. Teuben, Organometallics 1991, 10, 3246;
- 12iM. Booij, B.-J. Deelman, R. Duchateau, D. S. Postma, A. Meetsma, J. H. Teuben, Organometallics 1993, 12, 3531;
- 12jB.-J. Deelman, W. M. Stevels, J. H. Teuben, M. T. Lakin, A. L. Spek, Organometallics 1994, 13, 3881;
- 12kB.-J. Deelman, M. Booij, A. Meetsma, J. H. Teuben, H. Kooijman, A. L. Spek, Organometallics 1995, 14, 2306;
- 12lR. Duchateau, C. T. van Wee, J. H. Teuben, Organometallics 1996, 15, 2291;
- 12mN. S. Radu, S. L. Buchwald, B. Scott, C. J. Burns, Organometallics 1996, 15, 3913;
- 12nS. Arndt, T. P. Spaniol, J. Okuda, Eur. J. Inorg. Chem. 2001, 73;
- 12oS. Arndt, A. Trifonov, T. P. Spaniol, J. Okuda, M. Kitamura, T. Takahashi, J. Organomet. Chem. 2002, 647, 158;
- 12pT. P. Spaniol, J. Okuda, M. Kitamura, T. Takahashi, J. Organomet. Chem. 2003, 684, 194.
- 13
- 13aA. D. Sadow, T. D. Tilley, Angew. Chem. 2003, 115, 827;
10.1002/ange.200390182 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 803;
- 13bA. D. Sadow, T. D. Tilley, J. Am. Chem. Soc. 2003, 125, 7971;
- 13cA. D. Sadow, T. D. Tilley, J. Am. Chem. Soc. 2005, 127, 643;
- 13dF.-G. Fontaine, T. D. Tilley, Organometallics 2005, 24, 4340.
- 14For examples of half-sandwich rare-earth-catalyzed organic synthesis, see:
- 14aW.-X. Zhang, Z. Hou, Org. Biomol. Chem. 2008, 6, 1720;
- 14bM. Nishiura, Z. Hou, Bull. Chem. Soc. Jpn. 2010, 83, 595;
- 14cV. M. Arredondo, S. Tian, F. E. McDonald, T. J. Marks, J. Am. Chem. Soc. 1999, 121, 3633;
- 14dS. Tian, V. M. Arredondo, C. L. Stern, T. J. Marks, Organometallics 1999, 18, 2568;
- 14eM. R. Douglass, T. J. Marks, J. Am. Chem. Soc. 2000, 122, 1824;
- 14fA. A. Trifonov, T. P. Spaniol, J. Okuda, Organometallics 2001, 20, 4869;
- 14gM. Nishiura, Z. Hou, Y. Wakatsuki, T. Yamaki, T. Miyamoto, J. Am. Chem. Soc. 2003, 125, 1184;
- 14hO. Tardif, M. Nishiura, Z. Hou, Tetrahedron 2003, 59, 10525;
- 14iW.-X. Zhang, M. Nishiura, Z. Hou, J. Am. Chem. Soc. 2005, 127, 16788;
- 14jW.-X. Zhang, M. Nishiura, Z. Hou, Synlett 2006, 1213;
- 14kY. Liu, M. Nishiura, Y. Wan, Z. Hou, J. Am. Chem. Soc. 2006, 128, 8124;
- 14lW.-X. Zhang, M. Nishiura, Z. Hou, Chem. Eur. J. 2007, 13, 4037;
- 14mW.-X. Zhang, M. Nishiura, T. Mashiko, Z. Hou, Chem. Eur. J. 2008, 14, 2167;
- 14nW.-X. Zhang, M. Nishiura, Z. Hou, Angew. Chem. 2008, 120, 9846; Angew. Chem. Int. Ed. 2008, 47, 9700.
- 15The reaction of 1 f with PhSiH3 to give a Sc silyl species was not observed.
- 16A previously reported, analogous PMe3-coordinated binuclear scandium hydride complex: P. J. Shapiro, E. Bunel, W. P. Schaefer, J. E. Bercaw, Organometallics 1990, 9, 867.
- 17For examples of hydride formation by the reaction of rare-earth alkyls with hydrosilanes, see: G. A. Molander, A. C. Romero, Chem. Rev. 2002, 102, 2161.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.