Alicyclic Ring Structure: Conformational Influence of the CF2 Group in Cyclododecanes†
Maciej Skibinski
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/group
Search for more papers by this authorDr. Yi Wang
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/group
Search for more papers by this authorProf. Alexandra M. Z. Slawin
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/group
Search for more papers by this authorDr. Tomas Lebl
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/group
Search for more papers by this authorProf. Dr. Peer Kirsch
Merck KGaA, PM-A Emerging Technologies Germany, Frankfurter Str. 250, 64293 Darmstadt (Germany)
Search for more papers by this authorCorresponding Author
Prof. David O'Hagan
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/group
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/groupSearch for more papers by this authorMaciej Skibinski
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/group
Search for more papers by this authorDr. Yi Wang
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/group
Search for more papers by this authorProf. Alexandra M. Z. Slawin
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/group
Search for more papers by this authorDr. Tomas Lebl
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/group
Search for more papers by this authorProf. Dr. Peer Kirsch
Merck KGaA, PM-A Emerging Technologies Germany, Frankfurter Str. 250, 64293 Darmstadt (Germany)
Search for more papers by this authorCorresponding Author
Prof. David O'Hagan
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/group
EaStChem School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST (UK) http://chemistry.st-and.ac.uk/staff/doh/groupSearch for more papers by this authorD.O’H. thanks the ERC for an Advanced Investigator Grant to support this research.
Graphical Abstract
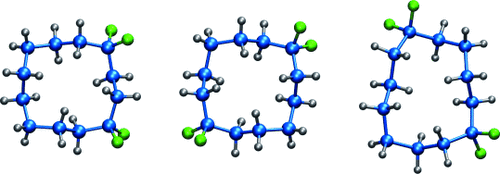
Man geht sich aus dem Weg: In fluorierten Cyclododecanen nimmt die CF2-Gruppe ausschließlich Eckpositionen ein (siehe Bild; blau C, grau H, grün F), wodurch transannulare 1,4-H,H-Wechselwirkungen infolge einer Aufweitung des C-CF2-C-Winkels relaxieren. Fehlgeordnete CF2-Gruppen verursachen hingegen eine signifikante Verzerrung der Ringstruktur. Der Einbau von CF2-Gruppen könnte daher als Designelement zur Einführung von Ordnung und Polarität in organische Kohlenwasserstoffstrukturen dienen.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201105060_sm_miscellaneous_information.pdf876.1 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. Kirsch, Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, 2004;
10.1002/352760393X Google Scholar
- 1bR. D. Chambers, Fluorine in Organic Chemistry, Blackwell, Oxford, 2004.
10.1002/9781444305371 Google Scholar
- 2
- 2aS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 2bK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881–1886.
- 3D. O’Hagan, Chem. Soc. Rev. 2008, 37, 308–319.
- 4
- 4aI. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Oxford, 2009;
10.1002/9781444312096 Google Scholar
- 4bA. Tressaud, G. Haufe, Fluorine in Health, Elsevier, Amsterdam, 2008;
- 4cA. M. Thayer, Chem. Eng. News 2006, 84, 15–24.
- 5
- 5aV. N. Boiko, Beilstein J. Org. Chem. 2010, 6, 880–921;
- 5bF. Leroux, P. Jeschke, M. Schlosser, Chem. Rev. 2005, 105, 827–856;
- 5cP. Jeschke, ChemBioChem 2004, 5, 570–589.
- 6
- 6aP. Kirsch, M. Bremer, ChemPhysChem 2010, 11, 357–360;
- 6bP. Kirsch, W. Binder, A. Hahn, K. Jahrling, M. Lenges, L. Lietzau, D. Maillard, V. Meyer, E. Poetsch, A. Ruhl, G. Unger, R. Frohlich, Eur. J. Org. Chem. 2008, 3479–3487.
- 7
- 7aT. Ema, T. Kadoya, K. Akihara, T. Sakai, J. Mol. Catal. B 2010, 66, 198–202;
- 7bN. Liu, S. Cao, L. Shen, J. Wu, J. Yu, J. Zhang, H. Li, X. Qian, Tetrahedron Lett. 2009, 50, 1982–1985.
- 8
- 8aD. Papoušek, Z. Papoušková, D. P. Chong, J. Phys. Chem. 1995, 99, 15387–15395;
- 8bG. L. Fox, H. B. Schlegel, J. Chem. Phys. 1990, 92, 4351–4357.
- 9A search of the Cambridge Crystallographic Data Centre (CCDC) was carried out using the ConQuest Software.
- 10K. B. Wiberg, P. R. Rablen, J. Am. Chem. Soc. 1993, 115, 625–631.
- 11R. J. Gillespie, E. A. Robinson, Chem. Soc. Rev. 2005, 34, 396–407.
- 12J. D. Dunitz, H. M. M. Shearer, Helv. Chim. Acta 1960, 43, 18–35. See also a commentary in H.-B. Bürgi, J. D. Dunitz, Helv. Chim. Acta 1993, 76, 1115–1166.
- 13
- 13aThe [3333] nomenclature comes from J. Dale, Acta Chem. Scand. 1973, 27, 1115–1129;
- 13bE. L. Eliel, S. H. Wilen, Stereochemistry of Organic Compounds, Wiley-Interscience, New York, 1994.
- 14F. A. L. Anet, T. N. Rawdah, J. Am. Chem. Soc. 1978, 100, 7166–7170.
- 15E. G. Atavin, V. S. Mastryukov, N. L. Allinger, A. Almenningen, R. Seip, J. Mol. Struct. 1989, 212, 87–95.
- 16F. A. L. Anet, A. K. Cheng, J. J. Wagner, J. Am. Chem. Soc. 1972, 94, 9250–9252.
- 17D. R. Strobach, G. A. Boswell, J. Org. Chem. 1971, 36, 818–820.
- 18Gaussian 03, Revision D.01: M. J. Frisch et al. (see Supporting Information), Gaussian, Inc., Wallingford CT, 2004. The minimum geometries were optimized on the B3LYP/6-311+G(2d,p) level of theory, and were verified to have only positive eigenfrequencies. The energies of the conformers were calculated on the MP2/6–311+G(2d,p) level of theory, using the B3LYP/6-311+G(2d,p) geometries and zero point energies.
- 19S. C. Sondej, J. A. Katzenellenbogen, J. Org. Chem. 1986, 51, 3508–3513.
- 20H. Eyring, J. Chem. Phys. 1935, 3, 107–115.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.