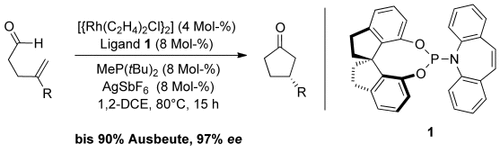
Catalytic Asymmetric Intramolecular Hydroacylation with Rhodium/Phosphoramidite–Alkene Ligand Complexes†
Dr. Thomas J. Hoffman
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland) http://www.carreira.ethz.ch
Search for more papers by this authorCorresponding Author
Prof. Dr. Erick M. Carreira
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland) http://www.carreira.ethz.ch
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland) http://www.carreira.ethz.chSearch for more papers by this authorDr. Thomas J. Hoffman
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland) http://www.carreira.ethz.ch
Search for more papers by this authorCorresponding Author
Prof. Dr. Erick M. Carreira
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland) http://www.carreira.ethz.ch
Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich (Switzerland) http://www.carreira.ethz.chSearch for more papers by this authorWe thank Dr. Marc LaFrance for his assistance in ligand synthesis.
Graphical Abstract
Zur Synthese funktionalisierter Cyclopentanone in guten Ausbeuten und mit hohen Enantioselektivitäten wurde eine asymmetrische intramolekulare Rh-katalysierte Hydroacylierung von Pent-4-enalen entwickelt (siehe Schema; DCE=1,2-Dichlorethan). Dieser Prozess nutzt Rh-Komplexe mit neuartigen modularen Phosphoramidit-Alken-Liganden und achiralen Phosphin-Coliganden.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201104595_sm_miscellaneous_information.pdf1.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on chiral ligands featuring alkenes, see:
- 1aF. Glorius, Angew. Chem. 2004, 116, 3444; Angew. Chem. Int. Ed. 2004, 43, 3364;
- 1bC. Defieber, H. Grützmacher, E. M. Carreira, Angew. Chem. 2008, 120, 4558;
10.1002/ange.200703612 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4482;
- 1cJ. B. Johnson, T. Rovis, Angew. Chem. 2008, 120, 852;
10.1002/ange.200700278 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 840;
- 1dR. Shintani, T. Hayashi, Aldrichimica Acta 2009, 42, 31.
- 2For leading references on chiral diene ligands, see:
- 2aT. Hayashi, K. Ueyama, N. Tokunaga, K. Yoshida, J. Am. Chem. Soc. 2003, 125, 11508;
- 2bC. Fischer, C. Defieber, T. Suzuki, E. M. Carreira, J. Am. Chem. Soc. 2004, 126, 1628;
- 2cC. Defieber, J.-F. Paquin, S. Serna, E. M. Carreira, Org. Lett. 2004, 6, 3873;
- 2dN. Tokunaga, Y. Otomaru, K. Ueyama, R. Shintani, T. Hayashi, J. Am. Chem. Soc. 2004, 126, 13584;
- 2eY. Otomaru, N. Tokunaga, R. Shintani, T. Hayashi, Org. Lett. 2005, 7, 307;
- 2fY. Otomaru, A. Kina, R. Shintani, T. Hayashi, Tetrahedron: Asymmetry 2005, 16, 1673;
- 2gA. Kina, K. Ueyama, T. Hayashi, Org. Lett. 2005, 7, 5889;
- 2hG. Berthon-Gelloz, T. Hayashi, J. Org. Chem. 2006, 71, 8957;
- 2iZ.-Q. Wang, C.-G. Feng, M.-H. Xu, G.-Q. Lin, J. Am. Chem. Soc. 2007, 129, 5336;
- 2jS. Helbig, S. Sauer, N. Cramer, S. Laschat, A. Baro, W. Frey, Adv. Synth. Catal. 2007, 349, 2331;
- 2kK. Okamoto, T. Hayashi, V. H. Rawal, Org. Lett. 2008, 10, 4387;
- 2lC.-G. Feng, Z.-Q. Wang, P. Tian, M.-H. Xu, G.-Q. Lin, Chem. Asian J. 2008, 3, 1511;
- 2mM. K. Brown, E. J. Corey, Org. Lett. 2010, 12, 172;
- 2nC. Shao, H.-J. Yu, N.-Y. Wu, C.-G. Feng, G.-Q. Lin, Org. Lett. 2010, 12, 3820;
- 2oY. Luo, A. J. Carnell, Angew. Chem. 2010, 122, 2810; Angew. Chem. Int. Ed. 2010, 49, 2750;
- 2pZ. Cao, Y. Liu, Z. Liu, X. Feng, M. Zhuang, H. Du, Org. Lett. 2011, 13, 2164;
- 2qQ. Li, Z. Dong, Z.-X. Yu, Org. Lett. 2011, 13, 1122.
- 3For leading references on chiral phosphine–alkene ligands, see:
- 3aP. Maire, S. Deblon, F. Breher, J. Geier, C. Böhler, H. Rüegger, H. Schönberg, H. Grützmacher, Chem. Eur. J. 2004, 10, 4198;
- 3bM. A. Grundl, J. J. Kennedy-Smith, D. Trauner, Organometallics 2005, 24, 2831;
- 3cF. Läng, F. Breher, D. Stein, H. Grützmacher, Organometallics 2005, 24, 2997;
- 3dR. Shintani, W.-L. Duan, K. Okamoto, T. Hayashi, Tetrahedron: Asymmetry 2005, 16, 3400;
- 3eR. Shintani, W.-L. Duan, T. Nagano, A. Okada, T. Hayashi, Angew. Chem. 2005, 117, 4687;
10.1002/ange.200501305 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4611;
- 3fP. Kasák, V. B. Arion, M. Widhalm, Tetrahedron: Asymmetry 2006, 17, 3084;
- 3gP. Stepnicka, I. Cisarova, Inorg. Chem. 2006, 45, 8785;
- 3hW.-L. Duan, H. Iwamura, R. Shintani, T. Hayashi, J. Am. Chem. Soc. 2007, 129, 2130;
- 3iR. T. Stemmler, C. Bolm, Synlett 2007, 1365;
- 3jC. Defieber, M. A. Ariger, P. Moriel, E. M. Carreira, Angew. Chem. 2007, 119, 3200;
10.1002/ange.200700159 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3139;
- 3kR. Mariz, A. Briceño, R. Dorta, R. Dorta, Organometallics 2008, 27, 6605;
- 3lT. Minuth, M. M. K. Boysen, Org. Lett. 2009, 11, 4212;
- 3mZ. Liu, H. Du, Org. Lett. 2010, 12, 3054.
- 4For references featuring synthetic applications of chiral olefin ligands, see:
- 4aJ.-F. Paquin, C. Defieber, C. R. J. Stephenson, E. M. Carreira, J. Am. Chem. Soc. 2005, 127, 10850;
- 4bJ.-F. Paquin, C. R. J. Stephenson, C. Defieber, E. M. Carreira, Org. Lett. 2005, 7, 3821;
- 4cR. Shintani, K. Okamoto, T. Hayashi, Org. Lett. 2005, 7, 4757;
- 4dR. Shintani, K. Okamoto, Y. Otomaru, K. Ueyama, T. Hayashi, J. Am. Chem. Soc. 2005, 127, 54;
- 4eR. Shintani, A. Tsurusaki, K. Okamoto, T. Hayashi, Angew. Chem. 2005, 117, 3977;
10.1002/ange.200500843 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3909;
- 4fR. Shintani, W.-L. Duan, T. Hayashi, J. Am. Chem. Soc. 2006, 128, 5628;
- 4gF.-X. Chen, A. Kina, T. Hayashi, Org. Lett. 2006, 8, 341;
- 4hK. Aikawa, S. Akutagawa, K. Mikami, J. Am. Chem. Soc. 2006, 128, 12648;
- 4iE. Piras, F. Läng, H. Rüegger, D. Stein, M. Worle, H. Grützmacher, Chem. Eur. J. 2006, 12, 5849;
- 4jR. Shintani, Y Sannohe, T. Tsuji, T. Hayashi, Angew. Chem. 2007, 119, 7415;
10.1002/ange.200702586 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7277;
- 4kT. Gendrineau, O. Chuzel, H. Eijsberg, J.-P. Genet, S. Darses, Angew. Chem. 2008, 120, 7783;
10.1002/ange.200803230 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7669;
- 4lR. Shintani, Y. Tsutsumi, M. Nagaosa, T. Nishimura, T. Hayashi, J. Am. Chem. Soc. 2009, 131, 13588;
- 4mG. Pattison, G. Piraux, H. W. Lam, J. Am. Chem. Soc. 2010, 132, 14373;
- 4nT. Nishimura, J. Wang, M. Nagaosa, K. Okamoto, R. Shintani, F.-Y. Kwong, W.-Y Yu, A. S. C. Chan, T. Hayashi, J. Am. Chem. Soc. 2010, 132, 464;
- 4oZ. Cao, H. Du, Org. Lett. 2010, 12, 2602;
- 4pR. Shintani, T. Hayashi, Org. Lett. 2011, 13, 350.
- 5For studies of olefin ligands containing the dibenzo[b,f]-azepine motif, see:
- 5aS. Deblon, H. Grützmacher, H. Schönberg, WO 03/048175 A1, 2003;
- 5bG. Mora, S. van Zutphen, C. Thoumazet, X. F. Le Goff, L. Ricard, H. Grützmacher, P. Le Floch, Organometallics 2006, 25, 5528;
- 5cP. Maire, F. Breher, H. Schönberg, H. Grützmacher, Organometallics 2005, 24, 3207;
- 5dH. Grützmacher, T. Büttner, P. Maire, M. Ramseier, D. Scheschkewitz, T. Zweifel, DE 102004027771, 2006; EP 05011539.3.
- 6M. Roggen, E. M. Carreira, J. Am. Chem. Soc. 2010, 132, 11917.
- 7E. Drinkel, A. Briceño, R. Dorta, R. Dorta, Organometallics 2010, 29, 2503.
- 8For a comprehensive review on hydroacylation, see: M. C. Willis, Chem. Rev. 2010, 110, 725.
- 9K. Sakai, J. Ide, O. Oda, N. Nakamura, Tetrahedron Lett. 1972, 13, 1287.
- 10
- 10aC. F. Lochow, R. G. Miller, J. Am. Chem. Soc. 1976, 98, 1281;
- 10bR. E. Campbell, Jr., C. F. Lochow, K. P. Vora, J. Am. Chem. Soc. 1980, 102, 5824;
- 10cR. E. Campbell, Jr., R. G. Miller, J. Organomet. Chem. 1980, 186, C 27.
- 11R. C. Larock, K. Oertle, G. F. Potter, J. Am. Chem. Soc. 1980, 102, 190.
- 12
- 12aD. P. Fairlie, B. Bosnich, Organometallics 1988, 7, 936;
- 12bD. P. Fairlie, B. Bosnich, Organometallics 1988, 7, 946;
- 12cR. W. Barnhart, X. Wang, P. Noheda, S. H. Bergens, J. Whelan, B. Bosnich, J. Am. Chem. Soc. 1994, 116, 1821;
- 12dR. W. Barnhart, D. A. McMorran, B. Bosnich, Inorg. Chim. Acta 1997, 263, 1;
- 12eW. Barnhart, D. A. McMorran, B. Bosnich, Chem. Commun. 1997, 589.
- 13
- 13aK. Sakai, Y. Ishiguro, F. Funakoshi, K. Ueno, H. Suemune, Tetrahedron Lett. 1984, 25, 961;
- 13bY. Taura, M. Tanaka, F. Funakoshi, K. Sakai, Tetrahedron Lett. 1989, 30, 6349;
- 13cT. Taura, T. Masakuza, X.-M. Yu, F. Funakoshi, K. Sakai, Tetrahedron 1991, 47, 4879;
- 13dM. Fujio, M. Tanaka, X.-M. Yu, F. Funakoshi, K. Sakai, Chem. Lett. 1998, 881;
- 13eM. Tanaka, M. Imai, M. Fujio, E. Sakamoto, M. Takahashi, Y. Eto-Kato, X.-M. Yu, K. Funkakoshi, K. Sakai, H. Suemune, J. Org. Chem. 2000, 65, 5806;
- 13fM. Imai, M. Tanaka, H. Sumeune, Tetrahedron 2001, 57, 1205.
- 14For additional notable reports of intramolecular pentenal hydroacylation, see :
- 14aP. Marcé, Y. Díaz, M. I. Matheu, S. Castillón, Org. Lett. 2008, 10, 4735;
- 14bK. Kundu, J. V. MaCullagh, A. T. Morehead, Jr., J. Am. Chem. Soc. 2005, 127, 16042.
- 15
- 15aR. W. Barnhart, B. Bosnich, Organometallics 1995, 14, 4343;
- 15bB. Bosnich, Acc. Chem. Res. 1998, 31, 667;
- 15cI. F. D. Hyatt, H. K. Anderson, A. T. Morehead, Jr., A. L. Sargent, Organometallics 2008, 27, 135, and references therein.
- 16For a recent review of combinatorial transition-metal catalysis, see: M. T. Reetz, Angew. Chem. 2008, 120, 2592;
10.1002/ange.200704327 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2556.
- 17
- 17aM. T. Reetz, T. Sell, A. Meiswinkel, G. Mehler, DE-A 10247633.0, 2002;
- 17bM. T. Reetz, T. Sell, A. Meiswinkel, G. Mehler, Angew. Chem. 2003, 115, 814;
10.1002/ange.200390178 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 790;
- 17cM. T. Reetz, O. Bondarev, Angew. Chem. 2007, 119, 4607;
10.1002/ange.200700533 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4523.
- 18S. Tosaki, K. Hara, V. Gnanadesikan, H. Morimoto, S. Harada, M. Sugita, N. Yamagiwa, S. Matsunaga, M. Shibasaki, J. Am. Chem. Soc. 2006, 128, 11776.
- 19
- 19aJ. Long, J. Hu, X. Shen, B. Ji, K. Ding, J. Am. Chem. Soc. 2002, 124, 10;
- 19bK. Ding, Pure Appl. Chem. 2006, 78, 293.
- 20B. L. Feringa, Acc. Chem. Res. 2000, 33, 346.
- 21The absolute configuration of 2 a was determined by comparison of the optical rotation with reported data: {
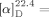 +26.4 (c=1.09, CH2Cl2); lit.:
+26.4 (c=1.09, CH2Cl2); lit.: 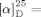 +36.7 (c=0.2, CH2Cl2)}, see Ref. [14a].
+36.7 (c=0.2, CH2Cl2)}, see Ref. [14a].
- 22Similar observations when using bicyclooctadiene ligands and Ph3P have been noted in Rh-catalyzed asymmetric cycloisomerizations of oxygen- and nitrogen-bridged 1,6-enynes, see: T. Nishimura, T. Kawamoto, M. Nagaosa, N. Kumamoto, T. Hayashi, Angew. Chem. 2010, 122, 1682;
10.1002/ange.200906792 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 1638.
- 23
- 23aJ. H. Zhang, J. Liao, K.-B. Yei, J. Zhu, J.-G. Deng, S.-F. Zhu, L.-X. Lin, Qi.-L. Zhou, L. W. Chung, T. Ye, Tetrahedron: Asymmetry 2002, 13, 1363;
- 23bV. B. Birmanm, A. L. Rheingold, K.-C. Lam, Tetrahedron: Asymmetry 1999, 10, 125.
- 24Additional phosphoramidite–alkene ligands are found in the Supporting Information.
- 25
- 25aF. López, A. J. Minnaard, B. L. Feringa, Acc. Chem. Res. 2007, 40, 179;
- 25bJ. F. Teichert, B. L. Feringa, Chem. Commun. 2011, 47, 2679, and references therein.
- 26
- 26aS. Oi, M. Moro, S. Ono, Y. Inoue, Chem. Lett. 1998, 83;
- 26bS. Oi, M. Moro, H. Ito, Y. Honma, S. Miyano, Y. Inoue, Tetrahedron 2002, 58, 91.
- 27
- 27aK. Soai, S. Yokoyama, T. Hayasaka, K. Ebihara, J. Org. Chem. 1988, 53, 4148;
- 27bA. Alexakis, Methodologies in Asymmetric Catalysis, American Chemical Society, Washington DC, 2004, chap. 4;
- 27cSee Ref. [30].
- 28
- 28aP. K. Fraser, S. Woodward, Chem. Eur. J. 2003, 9, 776;
- 28bA. Alexakis, V. Albrow, K. Biswas, M. d’Augustin, O. Prieto, S. Woodward, Chem. Commun. 2005, 284;
- 28cC. Hawner, K. Li, V. Cirriez, A. Alexakis, Angew. Chem. 2008, 120, 8334;
10.1002/ange.200803436 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8211.
- 29
- 29aE. Reyes, J. L. Vicario, L. Carrillo, D. Badía, U. Uria, A. Iza, J. Org. Chem. 2006, 71, 7763;
- 29bSee Ref. [30].
- 30For recent reviews on advances in enantioselective copper-catalyzed conjugate addition, see:
- 30aA. Alexakis, J. E. Bäckvall, N. Krause, O. Pàmies, M. Diéguez, Chem. Rev. 2008, 108, 2796;
- 30bT. Jerphagnon, M. G. Pizzuti, A. J. Minnaard, B. L. Feringa, Chem. Soc. Rev. 2009, 38, 1039.
- 31For recent reviews on advances in enantioselective conjugate addition of organoboron reagents, see:
- 31aT. Hayashi, K. Yamasaki, Chem. Rev. 2003, 103, 2829;
- 31bK. Fagnou, M. Lautens, Chem. Rev. 2003, 103, 169;
- 31cS. Darses, J.-P. Genêt, Eur. J. Org. Chem. 2003, 4313;
- 31dT. Hayashi, Bull. Chem. Soc. Jpn. 2004, 77, 13.
- 32
- 32aA. Hosomi, H. Sakurai, J. Am. Chem. Soc. 1977, 99, 1673;
- 32bS. Oi, Y. Honma, Y. Inoue, Org. Lett. 2002, 4, 667;
- 32cY. Nakao, J. Chen, H. Imanaka, T. Hiyama, Y. Ichikawa, W.-L. Duan, R. Shintani, T. Hayashi, J. Am. Chem. Soc. 2007, 129, 9137.
- 33P. A. Wender, S. T. Handy, D. L Wright, Chem. Ind. 1997, 765.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.