Ionic-Surfactant-Coated Burkholderia cepacia Lipase as a Highly Active and Enantioselective Catalyst for the Dynamic Kinetic Resolution of Secondary Alcohols†
Hyunjin Kim
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Search for more papers by this authorYoon Kyung Choi
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Search for more papers by this authorJusuk Lee
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Search for more papers by this authorEungyeong Lee
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Search for more papers by this authorCorresponding Author
Jaiwook Park
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)Search for more papers by this authorCorresponding Author
Prof. Mahn-Joo Kim
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)Search for more papers by this authorHyunjin Kim
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Search for more papers by this authorYoon Kyung Choi
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Search for more papers by this authorJusuk Lee
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Search for more papers by this authorEungyeong Lee
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Search for more papers by this authorCorresponding Author
Jaiwook Park
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)Search for more papers by this authorCorresponding Author
Prof. Mahn-Joo Kim
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)
Department of Chemistry, Pohang University of Science and Technology (POSTECH), San-31 Hyojadong, Pohang 790-784 (Republic of Korea)Search for more papers by this authorThis work was supported by the National Research Foundation of Korea (KRF-2007-511-C00031 and 2009-0074357).
Graphical Abstract
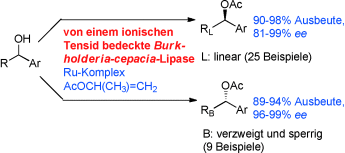
Aktiv durch Umhüllung: Das auf Burkholderia-cepacia-Lipase basierende Titelsystem ist hoch aktiv in der dynamischen kinetischen Racematspaltung (DKR). Seine wichtigsten Eigenschaften sind: die schnellste DKR von 1-Phenylethanol, die hoch enantioselektive DKR einer Vielzahl sekundärer Alkohole (RCH(OH)Ar) und das Schalten der Lipase-DKR-Enantioselektivität durch die Form der aliphatischen Kette (R).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201104141_sm_miscellaneous_information.pdf1.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. H. Lee, K. Han, M.-J. Kim, J. Park, Eur. J. Org. Chem. 2010, 999–1015;
- 1bY. Kim, J. Park, M.-J. Kim, ChemCatChem 2011, 3, 271–277;
- 1cH. Pellissier, Tetrahedron 2008, 64, 1563–1601; H. Pellissier, Tetrahedron 2011, 67, 3769–3802; H. Pellissier, Adv. Synth. Catal. 2011, 353, 659–676.
- 2
- 2a Hydrolases in Organic Synthesis, 2nd ed. ), Wiley-VCH, Weinheim, 2006;
- 2b Asymmetric Organic Synthesis with Enzymes (Eds.: ), Wiley-VCH, Weinheim, 2008.
- 3For DKR of alcohols, see:
- 3aS.-B. Ko, B. Baburaj, M.-J. Kim, J. Park, J. Org. Chem. 2007, 72, 6860–6864;
- 3bM.-J. Kim, Y. K. Choi, S. Kim, D. Kim, K. Han, S.-B. Ko, J. Park, Org. Lett. 2008, 10, 1295–1298;
- 3cR. M. Haak, F. Berthiol, T. Jerphagnon, J. A. Gayet, C. Tababiono, C. P. Postema, V. Ritleng, M. Pfeffer, D. B. Janssen, A. J. Minnaard, B. L. Feringa, J. G. De Vries, J. Am. Chem. Soc. 2008, 130, 13508–13509;
- 3dQ. Chen, C. Yuan, Chem. Commun. 2008, 5333–5335;
- 3eY. Do, I. C. Hwang, M.-J. Kim, J. Park, J. Org. Chem. 2010, 75, 5740–5742;
- 3fS. Akai, R. Hanada, N. Fujiwara, Y. Kita, M. Egi, Org. Lett. 2010, 12, 4900–4903;
- 3gL. K. Thalén, A. Sumic, K. Bogar, J. Norinder, A. K. A. Persson, J.-E. Bäckvall, J. Org. Chem. 2010, 75, 6842–6847.
- 4For DKR of amines, see:
- 4aM. A. J. Veld, K. Hult, A. R. A. Palmans, E. W. Meijer, Eur. J. Org. Chem. 2007, 5416–5421;
- 4bA. N. Parvulescu, P. A. Jacobs, D. E. De Vos, Chem. Eur. J. 2007, 13, 2034–2043;
- 4cM.-J. Kim, W.-H. Kim, K. Han, Y. K. Choi, J. Park, Org. Lett. 2007, 9, 1157–1159;
- 4dA. N. Parvulescu, P. A. Jacobs, D. E. De Vos, Adv. Synth. Catal. 2008, 350, 113–121;
- 4eK. Han, J. Park, M.-J. Kim, J. Org. Chem. 2008, 73, 4302–4304;
- 4fL. H. Andrade, A. V. Silva, E. C. Pedrozo, Tetrahedron Lett. 2009, 50, 4331–4334;
- 4gK. Han, Y. Kim, M.-J. Kim, Tetrahedron Lett. 2010, 51, 3536–3537;
- 4hY. Kim, M.-J. Kim, Tetrahedron Lett. 2010, 51, 5581–5584.
- 5The protein content was determined using the Bradford method. M. M. Bradford, Anal. Biochem. 1976, 72, 248–254.
- 6It is assumed that the high activity of ISCBCL may result from two effects of the coating: the protection of enzymes against deactivation during lyophilization and the better dispersion of enzymes in organic solvent. It may be also speculated that the coating could influence the enzymatic activity by changing the pH and water content of enzyme preparation. It was observed that the changes of the pH and water content by coating were not significant. The pH of BCL preparation increased slightly from 6.4 before coating to 6.8 after coating while the water content, determined by the Karl-Fisher titration, decreased from 11–12 % to 7–10 %.
- 7J. H. Lee, N. Kim, M.-J. Kim, J. Park, ChemCatChem 2011, 3, 354–359.
- 8
- 8aJ. H. Choi, Y. H. Kim, S. H. Nam, S. T. Shin, M.-J. Kim, J. Park, Angew. Chem. 2002, 114, 2479–2482;
Angew. Chem. Int. Ed. 2002, 41, 2373–2376;
10.1002/1521-3773(20020703)41:13<2373::AID-ANIE2373>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 8bJ. H. Choi, Y. K. Choi, Y. H. Kim, E. S. Park, E. J. Kim, M.-J. Kim, J. Park, J. Org. Chem. 2004, 69, 1972–1977.
- 9B. Martín-Matute, M. Edin, K. Bogár, F. B. Kaynak, J.-E. Bäckvall, J. Am. Chem. Soc. 2005, 127, 8817–8825.
- 10Novozym 435 accepts a limited range of secondary alcohols carrying a small and a significantly larger substituent at the hydroxymethine center with high enantioselectivity. In addition, the small substituent of secondary alcohol should be smaller than three-carbon unit for transformation at synthetically useful rate.
- 11It was observed from the NMR spectra of product mixtures that some of ionic surfactant in ISCBCL went into solution during the reaction.
- 12
- 12aR. J. Kazlauskas, A. N. E. Weissfloch, A. T. Rappaport, L. A. Cuccia, J. Org. Chem. 1991, 56, 2656–2665;
- 12bQ. Jing, R. J. Kazlauskas, Chirality 2008, 20, 724–735.
- 13Here, we used ISCBCL lyophilized in the presence of 13 wt % 4 in 1:1 (v/v) water–THF because more active ISCBCLs were developed later.
- 14This can be also observed with coating-free BCL, but the reactions are expected to be very slow. In a previous study, the resolution of 3 a with Lipase PS took 320 h to reach 51 % conversion at 25 °C (P. Allevi, P. Ciuffreda, M. Anastasia, Tetrahedron: Asymmetry 1997, 8, 93–99).
- 15This is compared to a low enantioselectivity (E=16) observed in the Lipase PS-catalyzed resolution described in Ref. [14].
- 16
- 16aT. Schulz, J. Pleiss, R. D. Schmid, Protein Sci. 2000, 9, 1053–1062;
- 16bD. Guieysse, C. Salagnad, P. Monsan, M. Remaud-Simeon, V. Tran, Tetrahedron: Asymmetry 2003, 14, 1807–1817.
- 17
- 17aS. Denmark, J. Fu, Chem. Rev. 2003, 103, 2763–2793;
- 17bL. Tietze, T. Kinzel, C. C. Brazel, Acc. Chem. Res. 2009, 42, 367–378;
- 17cB. M. Trost, A. H. Weiss, Adv. Synth. Catal. 2009, 351, 963–983.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.