A Membrane-Bound Antiparallel Dimer of Rat Islet Amyloid Polypeptide†
Dr. Abhinav Nath
Molecular Biophysics & Biochemistry, Yale University, New Haven CT 06520-8114 (USA)
Search for more papers by this authorCorresponding Author
Prof. Andrew D. Miranker
Molecular Biophysics & Biochemistry, Yale University, New Haven CT 06520-8114 (USA)
Molecular Biophysics & Biochemistry, Yale University, New Haven CT 06520-8114 (USA)Search for more papers by this authorCorresponding Author
Prof. Elizabeth Rhoades
Molecular Biophysics & Biochemistry, Yale University, New Haven CT 06520-8114 (USA)
Molecular Biophysics & Biochemistry, Yale University, New Haven CT 06520-8114 (USA)Search for more papers by this authorDr. Abhinav Nath
Molecular Biophysics & Biochemistry, Yale University, New Haven CT 06520-8114 (USA)
Search for more papers by this authorCorresponding Author
Prof. Andrew D. Miranker
Molecular Biophysics & Biochemistry, Yale University, New Haven CT 06520-8114 (USA)
Molecular Biophysics & Biochemistry, Yale University, New Haven CT 06520-8114 (USA)Search for more papers by this authorCorresponding Author
Prof. Elizabeth Rhoades
Molecular Biophysics & Biochemistry, Yale University, New Haven CT 06520-8114 (USA)
Molecular Biophysics & Biochemistry, Yale University, New Haven CT 06520-8114 (USA)Search for more papers by this authorThis work was funded by an American Heart Association Postdoctoral Fellowship for A.N. and NIH grant GM-084391 to E.R. and A.D.M. We thank Dr. S. G. Sligar (UIUC) for the gift of MSP1D1, Y. Gofman of the Ben-Tal group (Tel Aviv University) for assistance with MCPep, Dr. J. Knight, Dr. T. Craggs, and Dr. C. J. Wilson for critical reading, J. Dunn for expressed IAPP, the W. M. Keck Biotechnology Research Laboratory for peptide synthesis, and the Yale FAS High Performance Computing Center.
Graphical Abstract
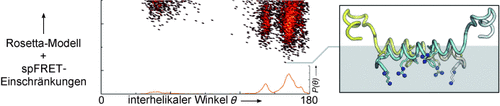
Aufschlussreich: Die Struktur eines antiparallelen Dimers des Inselamyloidpolypeptids der Ratte im Komplex mit anionischen Membrannanoscheibchen wurde durch Einzelpaar(sp)-FRET und Rosetta-Verfeinerung untersucht. Modelle des Dimers offenbarten eine mögliche Schnittstelle für die Lipidbindung und lassen vermuten, dass wesentliche Wechselwirkungen auch in der humanen Isoform auftreten. Die Befunde könnten Einblicke in die Fibrillenbildung bei Diabetes Typ II geben.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201102887_sm_miscellaneous_information.pdf697.6 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. A. Williamson, J. P. Loria, A. D. Miranker, J. Mol. Biol. 2009, 393, 383–396.
- 2P. Westermark, C. Wernstedt, E. Wilander, D. W. Hayden, T. D. O’Brien, K. H. Johnson, Proc. Natl. Acad. Sci. USA 1987, 84, 3881–3885.
- 3
- 3aJ. A. Hebda, A. D. Miranker, Annu. Rev. Biophys. 2009, 38, 125–152;
- 3bA. Abedini, D. P. Raleigh, Protein Eng. Des. Sel. 2009, 22, 453–459.
- 4J. D. Knight, J. A. Hebda, A. D. Miranker, Biochemistry 2006, 45, 9496–9508.
- 5J. R. Brender, E. L. Lee, M. A. Cavitt, A. Gafni, D. G. Steel, A. Ramamoorthy, J. Am. Chem. Soc. 2008, 130, 6424–6429.
- 6
- 6aS. M. Patil, S. Xu, S. R. Sheftic, A. T. Alexandrescu, J. Biol. Chem. 2009, 284, 11982–11991;
- 6bR. P. Nanga, J. R. Brender, J. Xu, K. Hartman, V. Subramanian, A. Ramamoorthy, J. Am. Chem. Soc. 2009, 131, 8252–8261;
- 6cR. P. Nanga, J. R. Brender, S. Vivekanandan, A. Ramamoorthy, Biochim. Biophys. Acta Biomembr. 2011, 1808, 2337–2342.
- 7
- 7aS. Luca, W. M. Yau, R. Leapman, R. Tycko, Biochemistry 2007, 46, 13505–13522;
- 7bS. A. Jayasinghe, R. Langen, J. Biol. Chem. 2004, 279, 48420–48425.
- 8
- 8aR. Mishra, M. Geyer, R. Winter, ChemBioChem 2009, 10, 1769–1772;
- 8bR. Soong, J. Brender, P. M. Macdonald, A. Ramamoorthy, J. Am. Chem. Soc. 2009, 131, 7079–7085;
- 8cF. Evers, C. Jeworrek, S. Tiemeyer, K. Weise, D. Sellin, M. Paulus, B. Struth, M. Tolan, R. Winter, J. Am. Chem. Soc. 2009, 131, 9516–9521;
- 8dS. Jha, D. Sellin, R. Seidel, R. Winter, J. Mol. Biol. 2009, 389, 907–920;
- 8eY. L. Ling, D. B. Strasfeld, S. H. Shim, D. P. Raleigh, M. T. Zanni, J. Phys. Chem. B 2009, 113, 2498–2505;
- 8fR. Kayed, J. Bernhagen, N. Greenfield, K. Sweimeh, H. Brunner, W. Voelter, A. Kapurniotu, J. Mol. Biol. 1999, 287, 781–796.
- 9L. Fu, G. Ma, E. C. Yan, J. Am. Chem. Soc. 2010, 132, 5405–5412.
- 10N. B. Last, E. Rhoades, A. D. Miranker, Proc. Natl. Acad. Sci. USA 2011, 108, 9460–9465.
- 11M. Magzoub, A. D. Miranker, unpublished results.
- 12M. Nishi, S. J. Chan, S. Nagamatsu, G. I. Bell, D. F. Steiner, Proc. Natl. Acad. Sci. USA 1989, 86, 5738–5742.
- 13A. Nath, A. J. Trexler, P. Koo, A. D. Miranker, W. M. Atkins, E. Rhoades, Methods Enzymol. 2010, 472, 89–117.
- 14
- 14aE. Andreetto, L. Yan, M. Tatarek-Nossol, A. Velkova, R. Frank, A. Kapurniotu, Angew. Chem. 2010, 122, 3146–3151;
10.1002/ange.200904902 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 3081–3085;
- 14bL. M. Yan, M. Tatarek-Nossol, A. Velkova, A. Kazantzis, A. Kapurniotu, Proc. Natl. Acad. Sci. USA 2006, 103, 2046–2051.
- 15
- 15aC. A. Rohl, C. E. Strauss, K. M. Misura, D. Baker, Methods Enzymol. 2004, 383, 66–93;
- 15bS. Chaudhury, S. Lyskov, J. J. Gray, Bioinformatics 2010, 26, 689–691;
- 15cK. W. Kaufmann, G. H. Lemmon, S. L. Deluca, J. H. Sheehan, J. Meiler, Biochemistry 2010, 49, 2987–2998.
- 16J. Williamson, A. Miranker, Protein Sci. 2007, 16, 110–117.
- 17D. Shental-Bechor, T. Haliloglu, N. Ben-Tal, Biophys. J. 2007, 93, 1858–1871.
- 18
- 18aR. Azriel, E. Gazit, J. Biol. Chem. 2001, 276, 34156–34161;
- 18bA. Fox, T. Snollaerts, C. Errecart Casanova, A. Calciano, L. A. Nogaj, D. A. Moffet, Biochemistry 2010, 49, 7783–7789.
- 19
- 19aN. F. Dupuis, C. Wu, J. E. Shea, M. T. Bowers, J. Am. Chem. Soc. 2009, 131, 18283–18292;
- 19bN. F. Dupuis, C. Wu, J. E. Shea, M. T. Bowers, J. Am. Chem. Soc. 2011, 133, 7240–7243.
- 20J. J. Wiltzius, S. A. Sievers, M. R. Sawaya, D. Eisenberg, Protein Sci. 2009, 18, 1521–1530.
- 21J. A. Hebda, I. Saraogi, M. Magzoub, A. D. Hamilton, A. D. Miranker, Chem. Biol. 2009, 16, 943–950.
- 22
- 22aS. Elbaum-Garfinkle, T. Ramlall, E. Rhoades, Biophys. J. 2010, 98, 2722–2730;
- 22bM. Morillas, W. Swietnicki, P. Gambetti, W. Surewicz, J. Biol. Chem. 1999, 274, 36859–36865.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.