Single Pyramid Magnets: Dy5 Pyramids with Slow Magnetic Relaxation to 40 K†
Dr. Robin J. Blagg
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598
Search for more papers by this authorDr. Christopher A. Muryn
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598
Search for more papers by this authorCorresponding Author
Prof. Eric J. L. McInnes
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598Search for more papers by this authorDr. Floriana Tuna
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598
Search for more papers by this authorCorresponding Author
Prof. Richard E. P. Winpenny
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598Search for more papers by this authorDr. Robin J. Blagg
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598
Search for more papers by this authorDr. Christopher A. Muryn
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598
Search for more papers by this authorCorresponding Author
Prof. Eric J. L. McInnes
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598Search for more papers by this authorDr. Floriana Tuna
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598
Search for more papers by this authorCorresponding Author
Prof. Richard E. P. Winpenny
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598
School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL (U.K.), Fax: (+44) 161-275-4598Search for more papers by this authorWe thank the EPSRC for funding and access to the Chemical Database Service at Daresbury.
Graphical Abstract
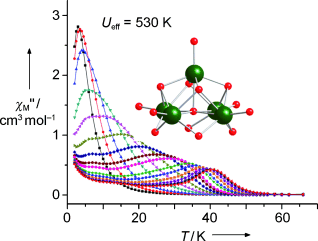
Einzelmolekülmagnete: Ein quadratisch-pyramidaler Dysprosium-Cluster mit fünf Metallzentren (siehe Bild) zeigte langsames magnetisches Relaxationsverhalten bei Temperaturen bis 40 K. Die thermische Energiebarriere für die Magnetisierungsrelaxation dieses Einzelmolekülmagneten ist 530 K; es ist damit die höchste Barriere, die für Clusterverbindungen der d- und f-Elemente bisher beobachtet wurde.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201101932_sm_miscellaneous_information.pdf201.2 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Sorace, C. Benelli, D. Gatteschi, Chem. Soc. Rev. 2011, DOI: .
- 2N. Ishikawa, M. Sugita, T. Ishikawa, S. Koshihara, Y. Kaizu, J. Am. Chem. Soc. 2003, 125, 8694–8695.
- 3
- 3aJ. K. Tang, I. Hewitt, N. T. Madhu, G. Chastanet, W. Wernsdorfer, C. E. Anson, C. Benelli, R. Sessoli, A. K. Powell, Angew. Chem. 2006, 118, 1761–1765; Angew. Chem. Int. Ed. 2006, 45, 1729–1733;
- 3bJ. Luzon, K. Bernot, I. J. Hewitt, C. E. Anson, A. K. Powell, R. Sessoli, Phys. Rev. Lett. 2008, 100, 247205.
- 4aI. J. Hewitt, J. Tang, N. T. Madhu, C. E. Anson, Y. Lan, J. Luzon, M. Etienne, R. Sessoli, A. K. Powell, Angew. Chem. 2010, 122, 6496–6500;
10.1002/ange.201002691 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6352–6356;
- 4bJ. D. Rinehart, M. Fang, W. J. Evans, J. R. Long, Nature Chem. 2011, DOI: .
- 5M. A. AlDamen, J. M. Clemente-Juan, E. Coronado, C. Martí-Gastaldo, A. Gaita-Ariño, J. Am. Chem. Soc. 2008, 130, 8874–8875.
- 6M. Kritikos, M. Moustiakimov, M. Wijk, G. Westin, J. Chem. Soc. Dalton Trans. 2001, 1931–1938.
- 7G. Helgesson, S. Jagner, O. Poncelet, L. G. Hubert-Pfalzgraf, Polyhedron 1991, 10, 1559–1564.
- 8G. Westin, M. Moustiakimov, M. Kritikos, Inorg. Chem. 2002, 41, 3249–3258.
- 9G. Westin, M. Kritikos, M. Wijk, J. Solid State Chem. 1998, 141, 168–176.
- 10D. C. Bradley, H. Chudzynska, D. M. Frigo, M. E. Hammond, M. B. Hursthouse, M. A. Mazid, Polyhedron 1990, 9, 719–726.
- 11S. Daniele, L. G. Hubert-Pfalzgraf, P. B. Hitchcock, M. F. Lappert, Inorg. Chem. Commun. 2000, 3, 218–222.
- 12J. Gromada, A. Mortreux, T. Chenal, J. W. Ziller, F. Leising, J.-F. Carpentier, Chem. Eur. J. 2002, 8, 3773–3788.
10.1002/1521-3765(20020816)8:16<3773::AID-CHEM3773>3.0.CO;2-S CAS PubMed Web of Science® Google Scholar
- 13Crystal data. For 1, C39H91Dy5O14, M=1596.62, monoclinic, space group C2, T=100(2) K, a=19.5597(6), b=16.4537(6), c=16.6240(5) Å, β=92.385(3)°, V=5345.5(3) Å3, Z=4, ρ=1.984 g cm−3, total data 17 428, unique data 8294 (Rint=0.0361), μ=6.956 mm−1, 644 parameters, 941 restraints, R1=0.0672 for I≥2σ(I) and wR2=0.2091 for all data. The data were recorded on a Oxford Xcalibur CCD diffractometer with MoKα radiation (λ=0.71073 Å). The structures were solved by direct methods and refined on F2 using SHELXTL. CCDC 817819 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14We have measured unit cells from multiple crystals from each batch crystallized and found each batch to contain a single phase.
- 15
- 15aK. S. Cole, R. H. Cole, J. Chem. Soc. 1941, 9, 341;
- 15bS. M. J. Aubin, Z. Sun, L. Pardi, J. Krzystek, K. Folting, L. J. Brunel, A. L. Rheingold, G. Christou, D. N. Hendrickson, Inorg. Chem. 1999, 38, 5329.
- 16C. J. Milios, A. Vinslava, W. Wernsdorfer, S. Moggach, S. Parsons, S. P. Perlepes, G. Christou, E. K. Brechin, J. Am. Chem. Soc. 2007, 129, 2754–2755.
- 17
- 17aN. Ishikawa, M. Sugita, N. Tanaka, T. Ishikawa, S.-Y. Koshihara, Y. Kaizu, Inorg. Chem. 2004, 43, 5498–5500;
- 17bS. Takamatsu, T. Ishikawa, S.-Y. Koshihara, N. Ishikawa, Inorg. Chem. 2007, 46, 7250–7252.
- 18
- 18aP.-H. Lin, T. J. Burchell, L. Ungur, L. F. Chibotaru, W. Wernsdorfer, M. Murugesu, Angew. Chem. 2009, 121, 9653–9656; Angew. Chem. Int. Ed. 2009, 48, 9489–9492;
- 18bJ. W. Sharples, Y.-Z. Zheng, F. Tuna, E. J. L. McInnes, D. Collison, Chem. Commun. 2011, DOI: .
- 19M. T. Gamer, Y. Lan, P. W. Roesky, A. K. Powell, R. Clérac, Inorg. Chem. 2008, 47, 6581–6583.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.