Macrocycle Size Matters: “Small” Functionalized Rotaxanes in Excellent Yield Using the CuAAC Active Template Approach†
Hicham Lahlali
School of Biological and Chemical Sciences, Queen Mary University of London, Joseph Priestley Building, Mile End Road, London, E1 4NS (UK)
Search for more papers by this authorKajally Jobe
School of Biological and Chemical Sciences, Queen Mary University of London, Joseph Priestley Building, Mile End Road, London, E1 4NS (UK)
Search for more papers by this authorMichael Watkinson
School of Biological and Chemical Sciences, Queen Mary University of London, Joseph Priestley Building, Mile End Road, London, E1 4NS (UK)
Search for more papers by this authorCorresponding Author
Dr. Stephen M. Goldup
School of Biological and Chemical Sciences, Queen Mary University of London, Joseph Priestley Building, Mile End Road, London, E1 4NS (UK)
School of Biological and Chemical Sciences, Queen Mary University of London, Joseph Priestley Building, Mile End Road, London, E1 4NS (UK)Search for more papers by this authorHicham Lahlali
School of Biological and Chemical Sciences, Queen Mary University of London, Joseph Priestley Building, Mile End Road, London, E1 4NS (UK)
Search for more papers by this authorKajally Jobe
School of Biological and Chemical Sciences, Queen Mary University of London, Joseph Priestley Building, Mile End Road, London, E1 4NS (UK)
Search for more papers by this authorMichael Watkinson
School of Biological and Chemical Sciences, Queen Mary University of London, Joseph Priestley Building, Mile End Road, London, E1 4NS (UK)
Search for more papers by this authorCorresponding Author
Dr. Stephen M. Goldup
School of Biological and Chemical Sciences, Queen Mary University of London, Joseph Priestley Building, Mile End Road, London, E1 4NS (UK)
School of Biological and Chemical Sciences, Queen Mary University of London, Joseph Priestley Building, Mile End Road, London, E1 4NS (UK)Search for more papers by this authorWe thank Majid Motevalli for assistance with the X-ray crystal structure of rotaxane 6. This work was supported by the Leverhulme Trust (Early Career Fellowship to S.M.G.), the Royal Society, and Q.M.U.L.. S.M.G. is a Royal Society Research Fellow. CuAAC=copper-catalyzed azide–alkyne cycloaddition.
Graphical Abstract
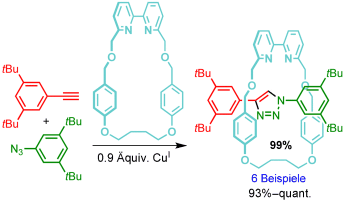
Größer ist nicht immer besser: Für die Azid-Alkin-Klickreaktion mit aktivem Templat wurde überraschend gefunden, dass kleinere Makrocyclen zu höheren Ausbeuten an [2]Rotaxan führen (siehe Schema). Der Ansatz wurde zur Synthese von „kleinen“ Rotaxanen mit potenziellen Anwendungen in der molekularen Elektronik, im Wirkstofftransport, in der Sensorik und in der enantioselektiven Katalyse genutzt.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201100415_sm_miscellaneous_information.pdf2.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aV. Aucagne, K. D. Hänni, D. A. Leigh, P. J. Lusby, D. B. Walker, J. Am. Chem. Soc. 2006, 128, 2186–2187;
- 1bS. Saito, E. Takahashi, K. Nakazono, Org. Lett. 2006, 8, 5133–5136;
- 1cJ. Berná, J. D. Crowley, S. M. Goldup, K. D. Hänni, A.-L. Lee, D. A. Leigh, Angew. Chem. 2007, 119, 5811–5815;
10.1002/ange.200701678 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5709–5713;
- 1dV. Aucagne, J. Berná, J. D. Crowley, S. M. Goldup, K. D. Hänni, D. A. Leigh, P. J. Lusby, V. E. Ronaldson, A. M. Z. Slawin, A. Viterisi, D. B. Walker, J. Am. Chem. Soc. 2007, 129, 11950–11963;
- 1eS. M. Goldup, D. A. Leigh, P. J. Lusby, R. T. McBurney, A. M. Z. Slawin, Angew. Chem. 2008, 120, 3429–3432;
10.1002/ange.200705859 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3381–3384;
- 1fJ. D. Crowley, K. D. Hänni, A.-L. Lee, D. A. Leigh, J. Am. Chem. Soc. 2007, 129, 12092–12093;
- 1gJ. Berná, S. M. Goldup, A.-L. Lee, D. A. Leigh, M. D. Symes, G. Teobaldi, F. Zerbetto, Angew. Chem. 2008, 120, 4464–4468;
10.1002/ange.200800891 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4392–4396;
- 1hY. Sato, R. Yamasaki, S. Saito, Angew. Chem. 2009, 121, 512–515; Angew. Chem. Int. Ed. 2009, 48, 504–507;
- 1iS. M. Goldup, D. A. Leigh, T. Long, P. R. McGonigal, M. D. Symes, J. Wu, J. Am. Chem. Soc. 2009, 131, 15924–15929;
- 1jJ. D. Crowley, S. M. Goldup, A.-L. Lee, D. A. Leigh, R. T. McBurney, Chem. Soc. Rev. 2009, 38, 1530–1541;
- 1kS. M. Goldup, D. A. Leigh, P. R. McGonigal, V. E. Ronaldson, A. M. Z. Slawin, J. Am. Chem. Soc. 2010, 132, 315–320;
- 1lJ. D. Crowley, K. D. Hänni, D. A. Leigh, A. M. Z. Slawin, J. Am. Chem. Soc. 2010, 132, 5309–5314;
- 1mJ. D. Crowley, S. M. Goldup, N. D. Gowans, D. A. Leigh, V. E. Ronaldson, A. M. Z. Slawin, J. Am. Chem. Soc. 2010, 132, 6243–6248;
- 1nS. M. Goldup, D. A. Leigh, R. T. McBurney, P. R. McGonigal, A. Plant, Chem. Sci. 2010, 1, 383–386.
- 2For selected recent reviews highlighting various aspects of, and strategies for, the passive template synthesis of interlocked molecules, see:
- 2aD. B. Amabilino, J. F. Stoddart, Chem. Rev. 1995, 95, 2725–2828;
- 2b Molecular Catenanes, Rotaxanes and Knots: A Journey Through the World of Molecular Topology (Eds.: ), Wiley-VCH, Weinheim, 1999;
- 2cG. A. Breault, C. A. Hunter, P. C. Mayers, Tetrahedron 1999, 55, 5265–5293;
- 2d Templated Organic Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2000;
- 2eT. J. Hubin, D. H. Busch, Coord. Chem. Rev. 2000, 200–202, 5–52;
- 2fK. Kim, Chem. Soc. Rev. 2002, 31, 96–107;
- 2gS. K. Menon, T. B. Guha, Y. K. Agrawal, Rev. Inorg. Chem. 2004, 24, 97–133;
- 2hF. Aricó, J. D. Badjic, S. J. Cantrill, A. H. Flood, K. C.-F. Leung, Y. Liu, J. F. Stoddart, Top. Curr. Chem. 2005, 249, 203–259;
- 2iO. Lukin, F. Vögtle, Angew. Chem. 2005, 117, 1480–1501;
10.1002/ange.200460312 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1456–1477;
- 2jC. Dietrich-Buchecker, B. X. Colasson, J.-P. Sauvage, Top. Curr. Chem. 2005, 249, 261–283;
- 2kE. Kay, D. Leigh, Top. Curr. Chem. 2005, 262, 133–177;
- 2lS. J. Loeb, Chem. Commun. 2005, 1511–1518;
- 2mA. Bogdan, Y. Rudzevich, M. O. Vysotsky, V. Bohmer, Chem. Commun. 2006, 2941–2952;
- 2nM. S. Vickers, P. D. Beer, Chem. Soc. Rev. 2007, 36, 211–225;
- 2oS. J. Loeb, Chem. Soc. Rev. 2007, 36, 226–235;
- 2pA. Vidonne, D. Philp, Tetrahedron 2008, 64, 8464–8475;
- 2qK. D. Hänni, D. A. Leigh, Chem. Soc. Rev. 2010, 39, 1240–1251.
- 3E. R. Kay, D. A. Leigh, F. Zerbetto, Angew. Chem. 2007, 119, 72–196; Angew. Chem. Int. Ed. 2007, 46, 72–191.
- 4Selected recent references:
- 4aV. Balzani, M. Clemente-León, A. Credi, B. Ferrer, M. Venturi, A. H. Flood, J. F. Stoddart, Proc. Natl. Acad. Sci. USA 2006, 103, 1178–1183;
- 4bJ. Wu, K. C.-F. Leung, D. Benítez, J.-Y. Han, S. J. Cantrill, L. Fang, J. F. Stoddart, Angew. Chem. 2008, 120, 7580–7584; Angew. Chem. Int. Ed. 2008, 47, 7470–7474;
- 4cM. J. Barrell, D. A. Leigh, P. J. Lusby, A. M. Z. Slawin, Angew. Chem. 2008, 120, 8156–8159;
10.1002/ange.200802745 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8036–8039;
- 4dS. Bonnet, J.-P. Collin, Chem. Soc. Rev. 2008, 37, 1207–1217;
- 4eJ. F. Stoddart, Chem. Soc. Rev. 2009, 38, 1802–1820;
- 4fV. Balzani, A. Credi, M. Venturi, Chem. Soc. Rev. 2009, 38, 1542–1550;
- 4gC.-F. Lee, D. A. Leigh, R. G. Pritchard, D. Schultz, S. J. Teat, G. A. Timco, R. E. P. Winpenny, Nature 2009, 458, 314–318;
- 4hS. Durot, F. Reviriego, J.-P. Sauvage, Dalton Trans. 2010, 39, 10557–10570;
- 4iJ. Berná, M. Alajarín, R.-A. Orenes, J. Am. Chem. Soc. 2010, 132, 10741–10747;
- 4jG. J. E. Davidson, S. Sharma, S. J. Loeb, Angew. Chem. 2010, 122, 5058–5062;
10.1002/ange.201001486 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 4938–4942;
- 4kA. I. Share, K. Parimal, A. H. Flood, J. Am. Chem. Soc. 2010, 132, 1665–1675.
- 5
- 5aD. A. Leigh, J. K. Y. Wong, F. Dehez, F. Zerbetto, Nature 2003, 424, 174–179;
- 5bJ. V. Hernández, E. R. Kay, D. A. Leigh, Science 2004, 306, 1532–1537.
- 6
- 6aM. N. Chatterjee, E. R. Kay, D. A. Leigh, J. Am. Chem. Soc. 2006, 128, 4058–4073;
- 6bV. Serreli, C.-F. Lee, E. R. Kay, D. A. Leigh, Nature 2007, 445, 523–527;
- 6cM. Alvarez-Pérez, S. M. Goldup, D. A. Leigh, A. M. Z. Slawin, J. Am. Chem. Soc. 2008, 130, 1836–1838.
- 7
- 7aC. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057–3062;
- 7bV. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708–2711;
10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2596–2599.10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 8
- 8aA. G. Cheetham, M. G. Hutchings, T. D. W. Claridge, H. L. Anderson, Angew. Chem. 2006, 118, 1626–1629;
10.1002/ange.200504064 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 1596–1599;
- 8bA. Fernandes, A. Viterisi, F. Coutrot, S. Potok, D. A. Leigh, V. Aucagne, S. Papot, Angew. Chem. 2009, 121, 6565–6569;
10.1002/ange.200903215 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6443–6447.
- 9
- 9aF. Cacialli, J. S. Wilson, J. J. Michels, C. Daniel, C. Silva, R. H. Friend, N. Severin, P. Samori, J. P. Rabe, M. J. O’Connell, P. N. Taylor, H. L. Anderson, Nat. Mater. 2002, 1, 160–164;
- 9bM. J. Frampton, H. L. Anderson, Angew. Chem. 2007, 119, 1046–1083;
10.1002/ange.200601780 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1028–1064;
- 9cJ. Terao, Y. Tanaka, S. Tsuda, N. Kambe, M. Taniguchi, T. Kawai, A. Saeki, S. Seki, J. Am. Chem. Soc. 2009, 131, 18046–18047.
- 10
- 10aC. M. S. Yau, S. I. Pascu, S. A. Odom, J. E. Warren, E. J. F. Klotz, M. J. Frampton, C. C. Williams, V. Coropceanu, M. K. Kuimova, D. Phillips, S. Barlow, J.-L. Bredas, S. R. Marder, V. Millar, H. L. Anderson, Chem. Commun. 2008, 2897–2899;
- 10bJ. J. Gassensmith, J. M. Baumes, B. D. Smith, Chem. Commun. 2009, 6329–6338;
- 10cJ. M. Baumes, J. J. Gassensmith, J. Giblin, J.-J. Lee, A. G. White, W. J. Culligan, W. M. Leevy, M. Kuno, B. D. Smith, Nat. Chem. 2010, 2, 1025–1030.
- 11
- 11aN. Kameta, Y. Nagawa, M. Karikomi, K. Hiratani, Chem. Commun. 2006, 3714–3716;
- 11bM. J. Chmielewski, J. J. Davis, P. D. Beer, Org. Biomol. Chem. 2009, 7, 415–424;
- 11cJ. J. Gassensmith, S. Matthys, J.-J. Lee, A. Wojcik, P. V. Kamat, B. D. Smith, Chem. Eur. J. 2010, 16, 2916–2921.
- 12Y. Tachibana, N. Kihara, T. Takata, J. Am. Chem. Soc. 2004, 126, 3438–3439.
- 13
- 13aY. Li, A. H. Flood, Angew. Chem. 2008, 120, 2689–2692; Angew. Chem. Int. Ed. 2008, 47, 2649–2652;
- 13bY. Hua, A. H. Flood, Chem. Soc. Rev. 2010, 39, 1262–1271.
- 14The crystal data and details of the structural refinement for rotaxane 6 are provided in the Supporting Information. CCDC 808340 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.