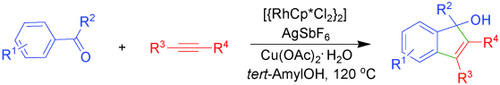Regioselective Synthesis of Indenols by Rhodium-Catalyzed CH Activation and Carbocyclization of Aryl Ketones and Alkynes†
Krishnamoorthy Muralirajan
Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan (China), Fax: (+886) 3572-4698 http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorDr. Kanniyappan Parthasarathy
Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan (China), Fax: (+886) 3572-4698 http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorCorresponding Author
Prof. Dr. Chien-Hong Cheng
Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan (China), Fax: (+886) 3572-4698 http://mx.nthu.edu.tw/%7Echcheng
Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan (China), Fax: (+886) 3572-4698 http://mx.nthu.edu.tw/%7EchchengSearch for more papers by this authorKrishnamoorthy Muralirajan
Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan (China), Fax: (+886) 3572-4698 http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorDr. Kanniyappan Parthasarathy
Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan (China), Fax: (+886) 3572-4698 http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorCorresponding Author
Prof. Dr. Chien-Hong Cheng
Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan (China), Fax: (+886) 3572-4698 http://mx.nthu.edu.tw/%7Echcheng
Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan (China), Fax: (+886) 3572-4698 http://mx.nthu.edu.tw/%7EchchengSearch for more papers by this authorWe thank the National Science Council of China (NSC-99-2811M-007-089) for support of this research.
Graphical Abstract
Keton und Alkin kommen zusammen: Die Titelreaktion verläuft bei 120 °C rasch und ist bereits nach einer Stunde abgeschlossen (siehe Schema). Die einzelnen Schritte sind vermutlich eine chelatgestützte ortho-C-H-Aktivierung, die Insertion der Alkineinheit, eine Carbocyclisierung und eine Protonierung. Cp*=Pentamethylcyclopentadienyl.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201100229_sm_miscellaneous_information.pdf3.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aKurakay Co., Ltd. Jpn. Kokai Tokkyo Koho, JP 81, 113, 740 (C1.C07C69/017), Sept 7, 1981 [Chem. Abstr. 1982, 96, 68724b];
- 1bKurakay Co., Ltd. Jpn. Kokai Tokkyo Koho, JP 82 04, 945 (C1. C07C69/013), Jan 11, 1982 [Chem. Abstr. 1982, 96, 199935u];
- 1cK. Samula, B. Cichy, Acta Pol. Pharm. 1985, 42, 256 [Chem. Abstr. 1986, 105, 171931v].
- 2
- 2aL. S. Liebeskind, J. R. Gasdaska, J. S. MaCallum, S. J. Tremont, J. Org. Chem. 1989, 54, 669;
- 2bR. C. Cambie, M. R. Metzler, P. S. Rutledge, P. D. Woodgate, J. Organomet. Chem. 1990, 381, C 26;
- 2cR. C. Cambie, M. R. Metzler, P. S. Rutledge, P. D. Woodgate, J. Organomet. Chem. 1990, 398, C 22;
- 2dN. P. Robinson, L. Main, B. K. Nicholson, J. Organomet. Chem. 1989, 364, C 37.
- 3
- 3aJ. Vicente, J.-A. Abad, J. G. Rubio, Organometallics 1996, 15, 3509;
- 3bJ. Vicente, J. A. Abad, B. Lopez-Pelages, E. Martinez-Puente, Organometallics 2002, 21, 58.
- 4
- 4aL. G. Quan, V. Gevorgyan, Y. Yamamoto, J. Am. Chem. Soc. 1999, 121, 3545;
- 4bL. G. Quan, V. Gevorgyan, Y. Yamamoto, J. Am. Chem. Soc. 1999, 121, 9485;
- 4cV. Gevorgyan, L. G. Quan, Y. Yamamoto, Tetrahedron Lett. 1999, 40, 4089.
- 5
- 5aD. K. Rayabarapu, C.-H. Cheng, Chem. Commun. 2002, 942;
- 5bD. K. Rayabarapu, C.-H. Yang, C.-H. Cheng, J. Org. Chem. 2003, 68, 6726;
- 5cK.-J. Chang, D. K. Rayabarapu, C.-H. Cheng, J. Org. Chem. 2004, 69, 4781.
- 6T. Matsuda, M. Makino, M. Murakami, Chem. Lett. 2005, 34, 1416.
- 7Metal-catalyzed carbocyclization:
- 7aD. B. Grotjahn in Comprehensive Organometallic Chemistry II, Vol. 12 (Ed.: ), Pergamon/Elsevier Science, Kidlington, 1995, pp. 703, 741;
10.1016/B978-008046519-7.00125-8 Google Scholar
- 7bM. Lautens, W. Klute, W. Tam, Chem. Rev. 1996, 96, 49;
- 7cI. Ojima, M. Tzamarioudaki, Z. R. Li, J. Donovan, Chem. Rev. 1996, 96, 635;
- 7dH. W. Frühauf, Chem. Rev. 1997, 97, 523.
- 8
- 8aF. Kakiuchi, T. Sato, F. Kakiuchi, N. Chatani, S. Murai, J. Mol. Catal. A 2002, 181–183, 511;
- 8bF. Kakiuchi, Y. Yamamoto, N. Chatani, S. Murai, Chem. Lett. 1995, 681;
- 8cK. Tsuchikama, M. Kasagawa, Y.-K. Hashimoto, K. Endo, T. Shibata, J. Organomet. Chem. 2008, 693, 3939;
- 8dS. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda, N. Chatani, Nature 1993, 366, 529;
- 8eR. Martinez, R. Chevalier, S. Darses, J. P. Genet. Angew. Chem. 2006, 118, 8412; Angew. Chem. Int. Ed. 2006, 45, 8232; Angew. Chem. Int. Ed. 2006, 45, 8232;
- 8fY. Ishii, N. Chatani, F. Kakiuchi, S. Murai, Tetrahedron Lett. 1997, 38, 7565;
- 8gP. W. R. Harris, C. E. F. Rickard, P. D. Woodgate, J. Organomet. Chem. 1999, 589, 168;
- 8hK. Tsuchikama, M. Kasagawa, K. Endo, T. Shibata, Synlett 2010, 97.
- 9
- 9aK. Parthasarathy, M. Jeganmohan, C.-H. Cheng, Org. Lett. 2008, 10, 325;
- 9bK. Parthasarathy, C.-H. Cheng, J. Org. Chem. 2009, 74, 9359;
- 9cV. S. Thirunavukkarasu, K. Parthasarathy, C.-H. Cheng, Angew. Chem. 2008, 120, 9604;
10.1002/ange.200804153 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9462;
- 9dV. S. Thirunavukkarasu, K. Parthasarathy, C.-H. Cheng, Chem. Eur. J. 2010, 16, 1436;
- 9eP. Gandeepan, K. Parthasarathy, C.-H. Cheng, J. Am. Chem. Soc. 2010, 132, 8569.
- 10
- 10aN. Umeda, H. Tsurugi, T. Satoh, M. Miura, Angew. Chem. 2008, 120, 4083;
10.1002/ange.200800924 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4019;
- 10bN. Guimond, K. Fagnou, J. Am. Chem. Soc. 2009, 131, 12050;
- 10cT. Fukutani, N. Umeda, K. Hirano, T. Satoh, M. Miura, Chem. Commun. 2009, 5141;
- 10dN. Guimond, C. Gouliaras, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 6908;
- 10eT. K. Hyster, T. Rovis, J. Am. Chem. Soc. 2010, 132, 10565;
- 10fS. Rakshit, F. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9585;
- 10gD. R. Stuart, P. Alsabeh, M. Kuhn, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 18326;
- 10hM. P. Huestis, L. Chan, D. R. Stuart, K. Fagnou, Angew. Chem. 2011, 123, 1374; Angew. Chem. Int. Ed. 2011, 50, 1338;
- 10iF. W. Patureau, T. Besset, N. Kuhl, F. Glorius, J. Am. Chem. Soc. 2011, 133, 2154;
- 10jZ.-M. Sun, S.-P. Chen, P. Zhao, Chem. Eur. J. 2010, 16, 2619;
- 10kD. N. Tran, N. Cramer, Angew. Chem. 2010, 122, 8357; Angew. Chem. Int. Ed. 2010, 49, 8181.
- 11For the optimization studies and the effect of the AgSbF6 salt on the yield of the reaction, see the Supporting Information.
- 12
- 12aC.-H. Jun, C. W. Moon, D.-Y. Lee, Chem. Eur. J. 2002, 8, 2422;
10.1002/1521-3765(20020603)8:11<2422::AID-CHEM2422>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 12bY. J. Park, C.-H. Jun, Bull. Korean Chem. Soc. 2005, 26, 877;
- 12cD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 12dT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 12eT. Satoh, M. Miura, Chem. Eur. J. 2010, 16, 11212.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.