Iridium-Catalyzed anti-Diastereo- and Enantioselective Carbonyl (α-Trifluoromethyl)allylation from the Alcohol or Aldehyde Oxidation Level†
Xin Gao
University of Texas at Austin, Department of Chemistry and Biochemistry, 1 University Station–A5300, Austin, TX 78712-1167 (USA), Fax: (+1) 512-471-8696
Search for more papers by this authorDr. Yong Jian Zhang
University of Texas at Austin, Department of Chemistry and Biochemistry, 1 University Station–A5300, Austin, TX 78712-1167 (USA), Fax: (+1) 512-471-8696
Search for more papers by this authorCorresponding Author
Prof. Michael J. Krische
University of Texas at Austin, Department of Chemistry and Biochemistry, 1 University Station–A5300, Austin, TX 78712-1167 (USA), Fax: (+1) 512-471-8696
University of Texas at Austin, Department of Chemistry and Biochemistry, 1 University Station–A5300, Austin, TX 78712-1167 (USA), Fax: (+1) 512-471-8696Search for more papers by this authorXin Gao
University of Texas at Austin, Department of Chemistry and Biochemistry, 1 University Station–A5300, Austin, TX 78712-1167 (USA), Fax: (+1) 512-471-8696
Search for more papers by this authorDr. Yong Jian Zhang
University of Texas at Austin, Department of Chemistry and Biochemistry, 1 University Station–A5300, Austin, TX 78712-1167 (USA), Fax: (+1) 512-471-8696
Search for more papers by this authorCorresponding Author
Prof. Michael J. Krische
University of Texas at Austin, Department of Chemistry and Biochemistry, 1 University Station–A5300, Austin, TX 78712-1167 (USA), Fax: (+1) 512-471-8696
University of Texas at Austin, Department of Chemistry and Biochemistry, 1 University Station–A5300, Austin, TX 78712-1167 (USA), Fax: (+1) 512-471-8696Search for more papers by this authorAcknowledgements is made to the Robert A. Welch Foundation (F-0038) and the NIH-NIGMS (RO1-GM069445). Y.J.Z. acknowledges partial financial support from Shanghai Jiao Tong University.
Graphical Abstract
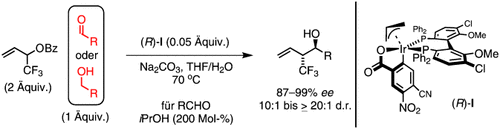
Florierendes Fluor: Die Umsetzung eines α-Trifluormethylallylbenzoats mit Alkoholen in Gegenwart eines ortho-cyclometallierten Iridium-Katalysators führt zur Bildung von Aldehyd-Allyliridium-Intermediaten, aus denen die enantiomerenangereicherten Produkte einer anti-α-Trifluormethylallylierung entstehen. Ein identischer Satz von Produkten wird ausgehend von Aldehyden erhalten, wenn ähnliche Transferhydrierungsbedingungen mit Isopropylalkohol als terminalem Reduktionsmittel angewendet werden.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201008296_sm_miscellaneous_information.pdf4.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1aA. M. Thayer, Chem. Eng. News 2006, 84, 15–24;
- 1bK. Mueller, C. Faeh, F. Diederich, Science 2007, 317, 1881–1886;
- 1cA. M. Thayer, Chem. Eng. News 2007, 85, 11–19.
- 2
- 2aJ. S. Carey, D. Laffan, C. Thomson, M. T. Williams, Org. Biomol. Chem. 2006, 4, 2337–2347;
- 2bV. Farina, J. T. Reeves, C. H. Senanayake, J. J. Song, Chem. Rev. 2006, 106, 2734–2793.
- 3For reviews on enantioselective fluorination, see:
- 3aJ.-A. Ma, D. Cahard, Chem. Rev. 2004, 104, 6119–6146;
- 3bV. A. Brunet, D. O’Hagan, Angew. Chem. 2008, 120, 1198–1201;
10.1002/ange.200704700 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1179–1182;
- 3cC. Bobbio, V. Gouverneur, Org. Biomol. Chem. 2006, 4, 2065–2075;
- 3dC. Audouard, J.-A. Ma, D. Cahard, Adv. Org. Synth. 2006, 2, 431–461;
- 3eJ.-A. Ma, D. Cahard, Chem. Rev. 2008, 108, PR 1–PR43;
- 3fL.-L. Cao, B. L. Gao, S.-T. Ma, Z.-P. Liu, Curr. Org. Chem. 2010, 14, 889–916;
- 3gY. Ku Kang, D. Y. Kim, Curr. Org. Chem. 2010, 14, 917–927;
- 3hD. Cahard, X. Xu, S. Couve-Bonnaire, X. Pannecoucke, Chem. Soc. Rev. 2010, 39, 558–568.
- 4For reviews on enantioselective trifluoromethylation, see:
- 4aJ.-A. Ma, D. Cahard, J. Fluorine Chem. 2007, 128, 975–996;
- 4bT. Billard, B. R. Langlois, Eur. J. Org. Chem. 2007, 891–897;
- 4cN. Shibata, S. Mizuta, H. Kawai, Tetrahedron: Asymmetry 2008, 19, 2633–2644;
- 4dJ. Nie, H.-C. Guo, D. Cahard, J.-A. Ma, Chem. Rev. 2011, 111, 455–529, and reference [3a].
- 5For aldehyde (trifluoromethyl)allylation and related processes, see:
- 5aT. Yamazaki, N. Ishikawa, Chem. Lett. 1984, 521–524;
- 5bY. Hanzawa, S. Ishizawa, Y. Kobayashi, T. Taguchi, Chem. Pharm. Bull. 1990, 38, 1104–1106;
- 5cT.-P. Loh, X.-R. Li, Angew. Chem. 1997, 109, 1029–1031; Angew. Chem. Int. Ed. Engl. 1997, 36, 980–982;
- 5dT.-P. Loh, X.-R. Li, Tetrahedron Lett. 1997, 38, 869–872;
- 5eT.-P. Loh, X.-R. Li, Eur. J. Org. Chem. 1999, 1893–1899;
10.1002/(SICI)1099-0690(199908)1999:8<1893::AID-EJOC1893>3.0.CO;2-3 CAS Web of Science® Google Scholar
- 5fT. Sakamoto, K. Takahashi, T. Yamazaki, T. Kitazume, J. Org. Chem. 1999, 64, 9467–9474;
- 5gQ. Chen, X.-L. Qiu, F.-L. Qing, J. Org. Chem. 2006, 71, 3762–3767.
- 6For selected reviews on enantioselective carbonyl allylation and crotylation, see:
- 6aR. W. Hoffmann, Angew. Chem. 1982, 94, 569–580; Angew. Chem. Int. Ed. Engl. 1982, 21, 555–566;
- 6bY. Yamamoto, N. Asao, Chem. Rev. 1993, 93, 2207–2293;
- 6cP. V. Ramachandran, Aldrichimica Acta 2002, 35, 23–35;
- 6dJ. W. J. Kennedy, D. G. Hall, Angew. Chem. 2003, 115, 4880–4887; Angew. Chem. Int. Ed. 2003, 42, 4732–4739;
- 6eS. E. Denmark, J. Fu, Chem. Rev. 2003, 103, 2763–2793;
- 6fC.-M. Yu, J. Youn, H.-K. Jung, Bull. Korean Chem. Soc. 2006, 27, 463–472;
- 6gI. Marek, G. Sklute, Chem. Commun. 2007, 1683–1691;
- 6hD. G. Hall, Synlett 2007, 1644–1655.
- 7For selected reviews on CC bond-forming transfer hydrogenation, see:
- 7aR. L. Patman, J. F. Bower, I. S. Kim, M. J. Krische, Aldrichimica Acta 2008, 41, 95–104;
- 7bF. Shibahara, M. J. Krische, Chem. Lett. 2008, 37, 1102–1107;
- 7cJ. F. Bower, I. S. Kim, R. L. Patman, M. J. Krische, Angew. Chem. 2009, 121, 36–48; Angew. Chem. Int. Ed. 2009, 48, 34–46;
- 7dS. B. Han, I. S. Kim, M. J. Krische, Chem. Commun. 2009, 7278–7287.
- 8For enantioselective carbonyl allylation by iridium catalyzed CC bond-forming transfer hydrogenation and related processes, see:
- 8aI. S. Kim, M.-Y. Ngai, M. J. Krische, J. Am. Chem. Soc. 2008, 130, 6340–6341;
- 8bI. S. Kim, M.-Y. Ngai, M. J. Krische, J. Am. Chem. Soc. 2008, 130, 14891–14899;
- 8cI. S. Kim, S.-B. Han, M. J. Krische, J. Am. Chem. Soc. 2009, 131, 2514–2520;
- 8dS. B. Han, I.-S. Kim, H. Han, M. J. Krische, J. Am. Chem. Soc. 2009, 131, 6916–6917;
- 8eY. Lu, I.-S. Kim, A. Hassan, D. J. Del Valle, M. J. Krische, Angew. Chem. 2009, 121, 5118–5121; Angew. Chem. Int. Ed. 2009, 48, 5018–5021;
- 8fJ. Itoh, S. B. Han, M. J. Krische, Angew. Chem. 2009, 121, 6431–6434; Angew. Chem. Int. Ed. 2009, 48, 6313–6316;
- 8gY. Lu, M. J. Krische, Org. Lett. 2009, 11, 3108–3111;
- 8hA. Hassan, Y. Lu, M. J. Krische, Org. Lett. 2009, 11, 3112–3115;
- 8iB. Bechem, R. L. Patman, S. Hashmi, M. J. Krische, J. Org. Chem. 2010, 75, 1795–1798;
- 8jS. B. Han, H. Han, M. J. Krische, J. Am. Chem. Soc. 2010, 132, 1760–1761;
- 8kY. J. Zhang, J. H. Yang, S. H. Kim, M. J. Krische, J. Am. Chem. Soc. 2010, 132, 4562–4563;
- 8lS. B. Han, X. Gao, M. J. Krische, J. Am. Chem. Soc. 2010, 132, 9153–9156;
- 8mJ. R. Zbieg, T. Fukuzumi, M. J. Krische, Adv. Synth. Catal. 2010, 352, 2416–2420.
- 9For selected examples of catalyst-directed diastereoselectivity, see:
- 9aN. Minami, S. S. Ko, Y. Kishi, J. Am. Chem. Soc. 1982, 104, 1109–1111;
- 9bS. Y. Ko, A. W. M. Lee, S. Masamune, L. A. Reed III, K. B. Sharpless, F. J. Walker, Science 1983, 220, 949–951;
- 9cS. Kobayashi, A. Ohtsubo, T. Mukaiyama, Chem. Lett. 1991, 831–834;
- 9dA. Hammadi, J. M. Nuzillard, J. C. Poulin, H. B. Kagan, Tetrahedron: Asymmetry 1992, 3, 1247–1262;
- 9eM. P. Doyle, A. V. Kalinin, D. G. Ene, J. Am. Chem. Soc. 1996, 118, 8837–8846;
- 9fB. M. Trost, T. L. Calkins, C. Oertelt, J. Zambrano, Tetrahedron Lett. 1998, 39, 1713–1716;
- 9gE. P. Balskus, E. N. Jacobsen, Science 2007, 317, 1736–1740;
- 9hS. B. Han, J. R. Kong, M. J. Krische, Org. Lett. 2008, 10, 4133–4135.
- 10O. K. Karjalainen, M. Passiniemi, A. M. P. Koskinen, Org. Lett. 2010, 12, 1145–1147.
- 11Q. Chen, F.-L. Qing, Tetrahedron 2007, 63, 11965–11972.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.