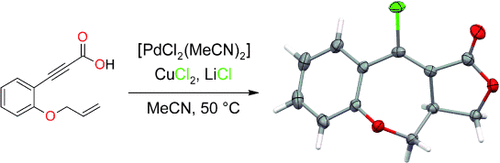Palladium-Catalyzed Intramolecular Carboesterification of Olefins†
We thank the University of Toronto, Canadian Foundation for Innovation, Ontario Ministry of Research and Innovation, and Natural Sciences and Engineering Research Council (NSERC) of Canada for funding. K.J.J. and R.T are grateful for NSERC and Ontario Graduate fellowships, respectively. Dr. Xiaodan Zhao and Prof. Shannon Stahl are thanked for helpful discussions.
Graphical Abstract
Ein Katalysator, drei Bindungen: Die Titelreaktion zwischen Propiolsäuren und nichtaktivierten Olefinen (siehe Schema; O rot, Cl grün) führt unter Bildung neuer C-C- und C-O-Bindungen zur vicinalen Funktionalisierung des Olefins. Strukturell komplexe tricyclische 6,7,5-Ringsysteme entstehen bei dieser Kaskade aus Chloropalladierung und formaler [3+2]-Cycloaddition in einem Schritt.