Chiral Ligand Design: A Bidentate Ligand Incorporating an Acyclic Phosphaalkene†
Julien Dugal-Tessier
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, BC, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847
Search for more papers by this authorGregory R. Dake Prof. Dr.
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, BC, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847
Search for more papers by this authorDerek P. Gates Prof. Dr.
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, BC, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847
Search for more papers by this authorJulien Dugal-Tessier
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, BC, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847
Search for more papers by this authorGregory R. Dake Prof. Dr.
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, BC, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847
Search for more papers by this authorDerek P. Gates Prof. Dr.
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, BC, V6T 1Z1 (Canada), Fax: (+1) 604-822-2847
Search for more papers by this authorWe gratefully acknowledge the following funding sources: The Natural Sciences and Engineering Research Council of Canada (NSERC Discovery and Research Tools grants to G.R.D. and D.P.G. and a PGS D scholarship to J.D.-T.), The Canada Foundation for Innovation, and The BC Knowledge Development Fund. We thank Joshua I. Bates for crystallographic work.
Graphical Abstract
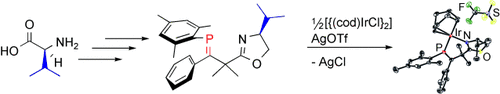
Einfach, aber stabil: Ein asymmetrisches Phosphaalken wurde aus L-Valin synthetisiert, einer leicht verfügbaren chiralen Vorstufe. Dieser luftstabile Ligand bildet einen P(sp2),N(sp2)-Komplex mit Iridium(I) (siehe Schema) und ist das erste Beispiel einer neuen Klasse von chiralen Phosphorliganden, die über ausgezeichnete π-Akzeptoreigenschaften verfügen sollten.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2008/z802949_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. Becker, Z. Anorg. Allg. Chem. 1976, 423, 242.
- 2See, for example:
- 2aK. B. Dillon, F. Mathey, J. F. Nixon, Phosphorus: The Carbon Copy, Wiley, Chichester, 1998;
- 2bF. Mathey, Angew. Chem. 2003, 115, 1616;
10.1002/ange.200200557 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1578;
- 2cL. Weber, Eur. J. Inorg. Chem. 2000, 2425;
- 2dR. Appel in Multiple Bonds and Low Coordination in Phosphorus Chemistry (Eds.: ), Thieme, Stuttgart, 1990.
- 3For recent reviews highlighting the use of low-coordinate phosphorus compounds in catalysis see:
- 3aP. Le Floch, Coord. Chem. Rev. 2006, 250, 627;
- 3bL. Weber, Angew. Chem. 2002, 114, 583; Angew. Chem. Int. Ed. 2002, 41, 563.
- 4For a recent review, see: C. Müller, D. Vogt, Dalton Trans. 2007, 5505; for recent work on mono- and diphosphinine complexes, see:
- 4aC. Müller, Z. Freixa, M. Lutz, A. L. Spek, D. Vogt, P. W. N. M. van Leeuwen, Organometallics 2008, 27, 834;
- 4bH. Lesnard, T. Cantat, P. Le Floch, I. Demachy, Y. Jean, Chem. Eur. J. 2007, 13, 2953;
- 4cO. Piechaczyk, T. Cantat, N. Mezailles, P. Le Floch, J. Org. Chem. 2007, 72, 4228;
- 4dC. Elschenbroich, J. Six, K. Harms, Chem. Commun. 2006, 3429;
- 4eC. Müller, L. G. Lopez, H. Kooijman, A. L. Spek, D. Vogt, Tetrahedron Lett. 2006, 47, 2017;
- 4fM. Doux, L. Ricard, P. Le Floch, Y. Jean, Organometallics 2006, 25, 1101;
- 4gP. Rosa, N. Mezailles, L. Ricard, F. Mathey, P. Le Floch, Y. Jean, Angew. Chem. 2001, 113, 1291;
10.1002/1521-3757(20010401)113:7<1291::AID-ANGE1291>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1251;10.1002/1521-3773(20010401)40:7<1251::AID-ANIE1251>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 4hB. Breit, R. Winde, T. Mackewitz, R. Paciello, K. Harms, Chem. Eur. J. 2001, 7, 3106;
10.1002/1521-3765(20010716)7:14<3106::AID-CHEM3106>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 4iP. Rosa, N. Mezailles, L. Ricard, F. Mathey, P. Le Floch, Angew. Chem. 2000, 112, 1893;
10.1002/(SICI)1521-3757(20000515)112:10<1893::AID-ANGE1893>3.0.CO;2-U Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1823.10.1002/(SICI)1521-3773(20000515)39:10<1823::AID-ANIE1823>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 5See, for example:
- 5aM. Ogasawara, A. Ito, K. Yoshida, T. Hayashi, Organometallics 2006, 25, 2715;
- 5bA. Suarez, C. W. Downey, G. C. Fu, J. Am. Chem. Soc. 2005, 127, 11244;
- 5cR. Shintani, G. C. Fu, Angew. Chem. 2003, 115, 4216;
10.1002/ange.200352103 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4082;
- 5dR. Shintani, G. C. Fu, J. Am. Chem. Soc. 2003, 125, 10778;
- 5eR. Shintani, G. C. Fu, Org. Lett. 2002, 4, 3699;
- 5fS. O. Agustsson, C. H. Hu, U. Englert, T. Marx, L. Wesemann, C. Ganter, Organometallics 2002, 21, 2993;
- 5gK. Tanaka, G. C. Fu, J. Org. Chem. 2001, 66, 8177;
- 5hM. Ogasawara, K. Yoshida, T. Hayashi, Organometallics 2001, 20, 3913;
- 5iK. Tanaka, S. Qiao, M. Tobisu, M. M. C. Lo, G. C. Fu, J. Am. Chem. Soc. 2000, 122, 9870;
- 5jX. Sava, L. Ricard, F. Mathey, P. Le Floch, Organometallics 2000, 19, 4899;
- 5kC. Ganter, C. Kaulen, U. Englert, Organometallics 1999, 18, 5444.
- 6J. Grundy, B. Donnadieu, F. Mathey, J. Am. Chem. Soc. 2006, 128, 7716.
- 7See, for example:
- 7aA. Hayashi, T. Yoshitomi, K. Umeda, M. Okazaki, F. Ozawa, Organometallics 2008, 27, 2321;
- 7bF. Ozawa, M. Yoshifuji, Dalton Trans. 2006, 4987;
- 7cH. Katayama, M. Nagao, T. Nishimura, Y. Matsui, K. Umeda, K. Akamatsu, T. Tsuruoka, H. Nawafune, F. Ozawa, J. Am. Chem. Soc. 2005, 127, 4350;
- 7dH. Z. Liang, S. Ito, M. Yoshifuji, Org. Lett. 2004, 6, 425;
- 7eA. S. Gajare, K. Toyota, M. Yoshifuji, F. Ozawa, Chem. Commun. 2004, 1994;
- 7fF. Ozawa, H. Okamoto, S. Kawagishi, S. Yamamoto, T. Minami, M. Yoshifuji, J. Am. Chem. Soc. 2002, 124, 10968;
- 7gT. Minami, H. Okamoto, S. Ikeda, R. Tanaka, F. Ozawa, M. Yoshifuji, Angew. Chem. 2001, 113, 4633;
10.1002/1521-3757(20011203)113:23<4633::AID-ANGE4633>3.0.CO;2-E Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4501;10.1002/1521-3773(20011203)40:23<4501::AID-ANIE4501>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 7hS. Ikeda, F. Ohhata, M. Miyoshi, R. Tanaka, T. Minami, F. Ozawa, M. Yoshifuji, Angew. Chem. 2000, 112, 4686; Angew. Chem. Int. Ed. 2000, 39, 4512.
- 8J. Dugal-Tessier, G. R. Dake, D. P. Gates, Organometallics 2007, 26, 6481.
- 9For recent work, see:
- 9aA. Hayashi, M. Okazaki, F. Ozawa, R. Tanaka, Organometallics 2007, 26, 5246;
- 9bF. Basuli, J. Tomaszewski, J. C. Huffman, D. J. Mindiola, J. Am. Chem. Soc. 2003, 125, 10170;
- 9cA. Ionkin, W. Marshall, Chem. Commun. 2003, 710;
- 9dA. Daugulis, M. Brookhart, P. S. White, Organometallics 2002, 21, 5935;
- 9eA. S. Ionkin, W. J. Marshall, Heteroat. Chem. 2002, 13, 662.
- 10B. Breit, J. Mol. Catal. A 1999, 143, 143.
- 11B. Breit, Chem. Commun. 1996, 2071.
- 12Mathey and co-workers have previously reported an acyclic L-menthyl-containing phosphaalkene as a [W(CO)5] complex at phosphorus (i.e. [(L-menthyl){(CO)5W}PCH(iPr)]. The free phosphaalkene was not reported. See, R. De Vaumas, A. Marinetti, L. Ricard, F. Mathey, J. Am. Chem. Soc. 1992, 114, 261.
- 13M. J. Mckennon, A. I. Meyers, K. Drauz, M. Schwarm, J. Org. Chem. 1993, 58, 3568.
- 14M. J. Kurth, O. H. W. Decker, H. Hope, M. D. Yanuck, J. Am. Chem. Soc. 1985, 107, 443.
- 15R. Luisi, V. Capriati, S. Florio, T. Vista, J. Org. Chem. 2003, 68, 9861.
- 16M. Yam, J. H. Chong, C. W. Tsang, B. O. Patrick, A. E. Lam, D. P. Gates, Inorg. Chem. 2006, 45, 5225.
- 17See, for example:
- 17aM. G. Schrems, E. Neumann, A. Pfaltz, Angew. Chem. 2007, 119, 8422;
10.1002/ange.200702555 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8274;
- 17bM. Engman, J. S. Diesen, A. Paptchikhine, P. G. Andersson, J. Am. Chem. Soc. 2007, 129, 4536;
- 17cM. Schelwies, P. Dübon, G. Helmchen, Angew. Chem. 2006, 118, 2526;
10.1002/ange.200503945 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2466;
- 17dD. J. Fox, S. B. Duckett, C. Flaschenriem, W. W. Brennessel, J. Schneider, A. Gunay, R. Eisenberg, Inorg. Chem. 2006, 45, 7197;
- 17eA. Alexakis, D. Polet, Org. Lett. 2004, 6, 3529.
- 18M. van der Sluis, V. Beverwijk, A. Termaten, E. Gavrilova, F. Bickelhaupt, H. Kooijman, N. Veldman, A. L. Spek, Organometallics 1997, 16, 1144.
- 19A. Hayashi, T. Ishiyama, M. Okazaki, F. Ozawa, Organometallics 2007, 26, 3708.
- 20CCDC 692226 (E-3 a) and 692227 (4) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 21Analysis of the Flack parameters for each structure [0.13(9) for E-3; 0.003(5) for 4] suggested that the data was refined on the correct enantiomer and that the compounds were close to enantiomeric purity.
- 22The PC bond lengths in dpcb complexes are also similar to those of the free ligand. See, for example: F. Ozawa, S. Kawagishi, T. Ishiyama, M. Yoshifuji, Organometallics 2004, 23, 1325. In addition, the 13C NMR spectroscopic data for the PC moiety [3 a: δ=203.3 ppm (1JPC=48 Hz); 4: δ=175.5 ppm (1JPC=58 Hz)] are consistent with that expected for a π-accepting ligand and are also observed in the case of the π-accepting dpcb. We speculate that the apparent contradiction in the expected PC bond length upon coordination may be a consequence of increased phosphorus s-orbital character in the PC σ-bond as evinced by the C-P-C angles.
- 23B. Schmid, L. M. Venanzi, T. Gerfin, V. Gramlich, F. Mathey, Inorg. Chem. 1992, 31, 5117.
- 24X. Sava, N. Mezailles, L. Ricard, F. Mathey, P. Le Floch, Organometallics 1999, 18, 807.
- 25
- 25aS. P. Smidt, F. Menges, A. Pfaltz, Org. Lett. 2004, 6, 2023;
- 25bP. Schnider, G. Koch, R. Prétôt, G. Z. Wang, F. M. Bohnen, C. Krüger, A. Pfaltz, Chem. Eur. J. 1997, 3, 887.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.