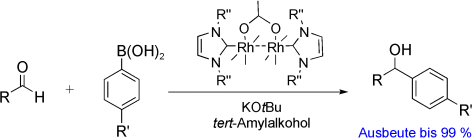
Tuning the Reactivity of Dirhodium(II) Complexes with Axial N-Heterocyclic Carbene Ligands: The Arylation of Aldehydes†
Pedro M. P. Gois Dr.
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Department of Chemistry, University College London, 20 Gordon Street, London WC1H OAJ, UK
Department of Chemistry, School of Life Sciences, University of Sussex, Falmer, Brighton BN1 9QJ, UK
Search for more papers by this authorAlexandre F. Trindade
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Search for more papers by this authorLuís F. Veiros Prof. Dr.
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Search for more papers by this authorVania André
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Search for more papers by this authorM. Teresa Duarte Prof. Dr.
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Search for more papers by this authorCarlos A. M. Afonso Prof. Dr.
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Search for more papers by this authorStephen Caddick Prof. Dr.
Department of Chemistry, University College London, 20 Gordon Street, London WC1H OAJ, UK
Search for more papers by this authorF. Geoffrey N. Cloke Prof. Dr.
Department of Chemistry, School of Life Sciences, University of Sussex, Falmer, Brighton BN1 9QJ, UK
Search for more papers by this authorPedro M. P. Gois Dr.
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Department of Chemistry, University College London, 20 Gordon Street, London WC1H OAJ, UK
Department of Chemistry, School of Life Sciences, University of Sussex, Falmer, Brighton BN1 9QJ, UK
Search for more papers by this authorAlexandre F. Trindade
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Search for more papers by this authorLuís F. Veiros Prof. Dr.
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Search for more papers by this authorVania André
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Search for more papers by this authorM. Teresa Duarte Prof. Dr.
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Search for more papers by this authorCarlos A. M. Afonso Prof. Dr.
CQFM and CQE, Departamento de Engenharia Química e Biológica, Complexo I, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal, Fax: (+351) 218-417-122
Search for more papers by this authorStephen Caddick Prof. Dr.
Department of Chemistry, University College London, 20 Gordon Street, London WC1H OAJ, UK
Search for more papers by this authorF. Geoffrey N. Cloke Prof. Dr.
Department of Chemistry, School of Life Sciences, University of Sussex, Falmer, Brighton BN1 9QJ, UK
Search for more papers by this authorThe Fundação para a Ciência e Tecnologia and FEDER (POCTI/QUI/60175/2004, POCI/QUI/58791/2004, SFRH/BPD/18624/2004, and SFRH/BD/30619/2006) are thanked for financial support.
Graphical Abstract
Effiziente Dimetallkatalysatoren: Ein Komplex von {Rh2(OAc)4} mit zwei N-heterocyclischen Carbenen (NHCs) in den axialen Positionen katalysiert die Arylierung von Aldehyden (siehe Schema; R=Alkyl, Aryl). Dichtefunktionalrechnungen für dieses System lassen erkennen, dass die Koordination der NHCs an den Dirhodium(II)-Komplex subtile stereoelektronische Effekte nach sich zieht. Als katalytisch aktive Spezies wird ein Komplex mit nur einem axialen NHC-Liganden vorgeschlagen.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z700924_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. P. Doyle, M. A. McKervey, T. Ye in Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley-Interscience, New York, 1998;
- 1bY. Lou, T. P. Remarchuk, E. J. Corey, J. Am. Chem. Soc. 2005, 127, 14223–14230;
- 1cF. A. Cotton, E. Hillard, C. A. Murillo, J. Am. Chem. Soc. 2002, 124, 5658–5660.
- 2
- 2aH. M. L. Davies, R. E. J. Beckwith, Chem. Rev. 2003, 103, 2861–2903;
- 2bM. P. Doyle, J. Org. Chem. 2006, 71, 9253–9260;
- 2cP. M. P. Gois, C. A. M. Afonso, Eur. J. Org. Chem. 2004, 3773–3788.
- 3Selected examples:
- 3aA. J. Catino, R. E. Forslund, M. P. Doyle, J. Am. Chem. Soc. 2005, 127, 13622–13623;
- 3bC. Liang, F. R. Peillard, C. Fruit, P. Müller, R. H. Dodd, P. Dauban, Angew. Chem. 2006, 118, 4757–4760; Angew. Chem. Int. Ed. 2006, 45, 4641–4644;
- 3cT. Washio, S. Nakamura, M. Anada, S. Hashimoto, Heterocycles 2005, 66, 567–578;
- 3dA. J. Catino, J. M. Nichols, R. E. Forslund, M. P. Doyle, Org. Lett. 2005, 7, 2787–2790.
- 4
- 4aF. E. Hahn, Angew. Chem. 2006, 118, 1374–1378; Angew. Chem. Int. Ed. 2006, 45, 1348–1352;
- 4bW. A. Herrmann, Angew. Chem. 2002, 114, 1342–1363;
Angew. Chem. Int. Ed. 2002, 41, 1290–1309;
10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 4c“N-Heterocyclic Carbenes in Transition Metal Catalysis”: Topics in Organometallic Chemistry, Vol. 21 (Ed.: ), Springer, Berlin/Heidelberg, 2007;
- 4d N-Heterocyclic Carbenes in Synthesis (Ed.: ), Wiley-VCH, Weinheim, 2006.
- 5
- 5aF. Schmidt, R. T. Stemmler, J. Rudolph, C. Bolm, Chem. Soc. Rev. 2006, 35, 454–470;
- 5bK. Fagnou, M. Lautens, Chem. Rev. 2003, 103, 169–196;
- 5cC. Bolm, J. P. Hildebrand, K. Muñiz, N. Hermanns, Angew. Chem. 2001, 113, 3382–3407;
Angew. Chem. Int. Ed. 2001, 40, 3284–3308.
10.1002/1521-3773(20010917)40:18<3284::AID-ANIE3284>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 6A. Fürstner, H. Krause, Adv. Synth. Catal. 2001, 343, 343–350.
- 7R. Dorta, E. D. Stevens, N. M. Scott, C. Costabile, L. Cavallo, C. D. Hoff, S. P. Nolan, J. Am. Chem. Soc. 2005, 127, 2485–2495.
- 8T. Hayashi, M. Takahashi, Y. Takaya, M. Ogasawara, J. Am. Chem. Soc. 2002, 124, 5052–5058.
- 9
- 9aM. Ueda, N. Miyaura, J. Org. Chem. 2000, 65, 4450–4452;
- 9bR. B. C. Jagt, J. G. de Vries, B. L. Feringa, A. Minnard, J. Org. Biomol. Chem. 2006, 4, 773–775.
- 10J. P. Snyder, A. Padwa, T. Stengel, A. J. Arduengo III, A. Jockisch, H.-L. Kim, J. Am. Chem. Soc. Soc. 2001, 123, 11318–11319.
- 11Crystallographic data for 20: C83H108N4O8Rh2, Mr=1495.55, orange crystals, 0.18×0.16×0.12 mm3, triclinic, space group P
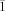 , a=12.6540(2), b=18.2140(3), c=19.1580(3) Å, α=103.5840(10), β=101.3890(10), γ=101.8950(10)°, V=4057.10(11) Å3, Z=2, T=150 K, ρcalcd=1.224 Mg m−3, μ=0.460 mm−1, F(000)=1576. For 21: C42H44N2O4Rh, Mr=743.7, orange crystals, 0.15×0.11×0.10 mm3, triclinic, space group P
, a=12.6540(2), b=18.2140(3), c=19.1580(3) Å, α=103.5840(10), β=101.3890(10), γ=101.8950(10)°, V=4057.10(11) Å3, Z=2, T=150 K, ρcalcd=1.224 Mg m−3, μ=0.460 mm−1, F(000)=1576. For 21: C42H44N2O4Rh, Mr=743.7, orange crystals, 0.15×0.11×0.10 mm3, triclinic, space group P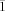 , a=11.256(2), b=12.538(3), c=15.367(5) Å, α=69.63(2), β=84.172(2), γ=77.614(3)°, V=1984.7(9) Å3, Z=2, T=150 K, ρcalcd=1.244 Mg m−3, μ=0.471 mm−1, F(000)=774. Data were collected with a Bruker AXS KAPPA APEX II diffractometer using graphite-monochromated MoKα radiation (λ=0.71069 Å). Structures were solved by direct methods (SIR97). Non-hydrogen atoms were refined anisotropically and hydrogen atoms were inserted in calculated positions as riding on the parent carbon atom (WINGX). Of 56 487 reflections for complex 20, 14 673 were independent (Rint=0.0492); 887 variables were refined to final R1(I>2σ(I))=0.0434, wR2(I>2σ(I))=0.0965, R1(all data)=0.0815, wR2(all data)=0.1065, GOF=1.037. For complex 21, of 30 833 reflections, 4408 were independent (Rint=0.0711); 411 variables were refined to final R1(I>2σ(I))=0.0675, wR2(I>2σ(I))=0.1714, R1(all data)=0.0945, wR2(all data)=0.2028, GOF=1.170. CCDC-639254 (20) and CCDC-644438 (21) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
, a=11.256(2), b=12.538(3), c=15.367(5) Å, α=69.63(2), β=84.172(2), γ=77.614(3)°, V=1984.7(9) Å3, Z=2, T=150 K, ρcalcd=1.244 Mg m−3, μ=0.471 mm−1, F(000)=774. Data were collected with a Bruker AXS KAPPA APEX II diffractometer using graphite-monochromated MoKα radiation (λ=0.71069 Å). Structures were solved by direct methods (SIR97). Non-hydrogen atoms were refined anisotropically and hydrogen atoms were inserted in calculated positions as riding on the parent carbon atom (WINGX). Of 56 487 reflections for complex 20, 14 673 were independent (Rint=0.0492); 887 variables were refined to final R1(I>2σ(I))=0.0434, wR2(I>2σ(I))=0.0965, R1(all data)=0.0815, wR2(all data)=0.1065, GOF=1.037. For complex 21, of 30 833 reflections, 4408 were independent (Rint=0.0711); 411 variables were refined to final R1(I>2σ(I))=0.0675, wR2(I>2σ(I))=0.1714, R1(all data)=0.0945, wR2(all data)=0.2028, GOF=1.170. CCDC-639254 (20) and CCDC-644438 (21) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 12F. H. Allen, Acta Crystallogr. Sect. B 2002, 58, 380–388.
- 13R. G. Parr, W. Yang in Density Functional Theory of Atoms and Molecules, Oxford University Press, New York, 1989.
- 14The optimizations were performed at the B3PW91/VDZP level with the Gaussian 98 package. Details and references are provided as Supporting Information.
- 15The calculated structures are depicted in the Supporting Information.
- 16Calculated as the difference between the energy of the two isolated fragments {Rh2(OAc)4} and NHC and the energy of the final species [Rh2(OAc)4(NHC)].
- 17
- 17aK. B. Wiberg, Tetrahedron 1968, 24, 1083;
- 17bWiberg indices are electronic parameters that are related to the electron density between atoms. They can be obtained from a natural population analysis and provide an indication of the bond strength.
- 18ORTEP3 for Windows: L. Farrugia, J. Appl. Crystallogr. 1997, 30, 565.
- 19All charges reported result from a natural population analysis. See the Supporting Information for details.
- 20
- 20aN. Yoshikai, E. Nakamura, Adv. Synth. Catal. 2003, 345, 1159–1171;
- 20bE. Nakamura, N. Yoshikai, M. Yamanaka, J. Am. Chem. Soc. 2002, 124, 7181–7192.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.