Porous Coordination Polymer with π Lewis Acidic Pore Surfaces, {[Cu3(CN)3{hat(CN)3(OEt)3}]⋅3 THF}n†
Daisuke Tanaka Dr.
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan, Fax: (+81) 75-383-2732
Search for more papers by this authorShigeyuki Masaoka Dr.
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan, Fax: (+81) 75-383-2732
Search for more papers by this authorSatoshi Horike
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan, Fax: (+81) 75-383-2732
Search for more papers by this authorShuhei Furukawa Dr.
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan, Fax: (+81) 75-383-2732
Search for more papers by this authorMotohiro Mizuno Prof. Dr.
Department of Chemistry, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa 920-1192, Japan
Search for more papers by this authorKazunaka Endo Prof. Dr.
Department of Chemistry, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa 920-1192, Japan
Search for more papers by this authorSusumu Kitagawa Prof. Dr.
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan, Fax: (+81) 75-383-2732
Search for more papers by this authorDaisuke Tanaka Dr.
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan, Fax: (+81) 75-383-2732
Search for more papers by this authorShigeyuki Masaoka Dr.
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan, Fax: (+81) 75-383-2732
Search for more papers by this authorSatoshi Horike
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan, Fax: (+81) 75-383-2732
Search for more papers by this authorShuhei Furukawa Dr.
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan, Fax: (+81) 75-383-2732
Search for more papers by this authorMotohiro Mizuno Prof. Dr.
Department of Chemistry, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa 920-1192, Japan
Search for more papers by this authorKazunaka Endo Prof. Dr.
Department of Chemistry, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa, Ishikawa 920-1192, Japan
Search for more papers by this authorSusumu Kitagawa Prof. Dr.
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan, Fax: (+81) 75-383-2732
Search for more papers by this authorhat=hexaazatriphenylene. This work was supported by a Grants-in-Aid for Scientific Research in a Priority Area “Chemistry of Coordination Space” (#434) and a Grant-in-Aid for Young Scientists (B) (#17750125) from the Ministry of Education, Culture, Sports, Science and Technology, Government of Japan.
Graphical Abstract
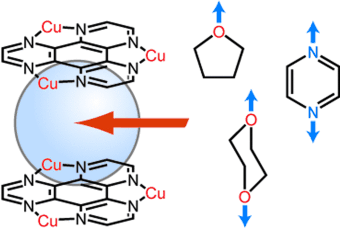
Neutrale organische Gastmoleküle sind in den Hohlräumen eines porösen Koordinationspolymers eingeschlossen. Dabei wechselwirken hauptsächlich elektronenarme π-Ebenen der Polymeroberfläche mit elektronegativen Atomen der Gastmoleküle (siehe Bild). Der thermogravimetrischen Analyse zufolge bilden Gastmoleküle mit zwei elektronegativen Atomen stabilere Komplexe mit dem Polymer als solche mit nur einem elektronegativen Atom.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2006/z600973_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. J. Langley, J. Hulliger, Chem. Soc. Rev. 1999, 28, 279–291;
- 1bB. Moulton, M. J. Zaworotko, Chem. Rev. 2001, 101, 1629–1658;
- 1cO. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi, J. Kim, Nature 2003, 423, 705–714;
- 1dG. S. Papaefstathiou, L. R. MacGillivray, Coord. Chem. Rev. 2003, 246, 169–184;
- 1eM. J. Rosseinsky, Microporous Mesoporous Mater. 2004, 73, 15–30;
- 1fR. J. Hill, D. L. Long, N. R. Champness, P. Hubberstey, M. Schröder, Acc. Chem. Res. 2005, 38, 337–350;
- 1gS. Kitagawa, R. Kitaura, S. Noro, Angew. Chem. 2004, 116, 2388–2430; Angew. Chem. Int. Ed. 2004, 43, 2334–2375.
- 2R. Matsuda, R. Kitaura, S. Kitagawa, Y. Kubota, R. V. Belosludov, T. C. Kobayashi, H. Sakamoto, T. Chiba, M. Takata, Y. Kawazoe, Y. Mita, Nature 2005, 436, 238–241.
- 3P. Sozzani, S. Bracco, A. Comotti, L. Ferretti, R. Simonutti, Angew. Chem. 2005, 117, 1850–1854;
10.1002/ange.200461704 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1816–1820.
- 4J. C. Ma, D. A. Dougherty, Chem. Rev. 1997, 97, 1303–1324.
- 5M. Nishio, CrystEngComm 2004, 6, 130–158.
- 6E. A. Meyer, R. K. Castellano, F. Diederich, Angew. Chem. 2003, 115, 1244–1287; Angew. Chem. Int. Ed. 2003, 42, 1210–1250.
- 7
- 7aD. Quinonero, C. Garau, C. Rotger, A. Frontera, P. Ballester, A. Costa, P. M. Deya, Angew. Chem. 2002, 114, 3539–3542;
10.1002/1521-3757(20020916)114:18<3539::AID-ANGE3539>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3389–3392;10.1002/1521-3773(20020916)41:18<3389::AID-ANIE3389>3.0.CO;2-S CAS PubMed Web of Science® Google Scholar
- 7bM. Mascal, A. Armstrong, M. D. Bartberger, J. Am. Chem. Soc. 2002, 124, 6274–6276;
- 7cY. Danten, T. Tassaing, M. Besnard, J. Phys. Chem. A 1999, 103, 3530–3534.
- 8For a recent experimental study on anion receptors with electron-deficient π systems, see:
- 8aR. M. Fairchild, K. T. Holman, J. Am. Chem. Soc. 2005, 127, 16364–16365;
- 8bS. Demeshko, S. Dechert, F. Meyer, J. Am. Chem. Soc. 2004, 126, 4508–4509;
- 8cY. S. Rosokha, S. V. Lindeman, S. V. Rosokha, J. K. Kochi, Angew. Chem. 2004, 116, 4750–4752;
10.1002/ange.200460337 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4650–4652;
- 8dP. de Hoog, P. Gamez, I. Mutikainen, U. Turpeinen, J. Reedijk, Angew. Chem. 2004, 116, 5939–5941;
10.1002/ange.200460486 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5815–5817;
- 8eO. B. Berryman, F. Hof, M. J. Hynes, D. W. Johnson, Chem. Commun. 2006, 506–508.
- 9M. Yoshizawa, T. Kusukawa, M. Kawano, T. Ohhara, I. Tanaka, K. Kurihara, N. Niimura, M. Fujita, J. Am. Chem. Soc. 2005, 127, 2798–2799.
- 10
- 10aB. E. Abrahams, P. A. Jackson, R. Robson, Angew. Chem. 1998, 110, 2801–2804;
10.1002/(SICI)1521-3757(19981002)110:19<2801::AID-ANGE2801>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2656–2659;10.1002/(SICI)1521-3773(19981016)37:19<2656::AID-ANIE2656>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 10bH. S. Grove, J. Julve, F. Lloret, Dalton Trans. 2001, 1029–1034;
- 10cM. Shatruk, A. Chouai, A. V. Prosvirin, K. R. Dunbar, Dalton Trans. 2005, 1897–1902;
- 10dX. H. Bu, K. Biradha, T. Yamaguchi, M. Nishimura, T. Ito, K. Tanaka, M. Shionoya, Chem. Commun. 2000, 1953–1954;
- 10eS. Kitagawa, S. Masaoka, Coord. Chem. Rev. 2003, 246, 73–88.
- 11
- 11aJ. T. Rademacher, A. W. Czarnik, J. Am. Chem. Soc. 1993, 115, 3018–3019;
- 11bE. O. Arikainen, N. Boden, R. J. Bushby, O. R. Lozman, J. G. Vinter, A. Wood, Angew. Chem. 2000, 112, 2423–2426;
10.1002/1521-3757(20000703)112:13<2423::AID-ANGE2423>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2333–2336;10.1002/1521-3773(20000703)39:13<2333::AID-ANIE2333>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 11cA. Frontera, F. Saczewski, M. Gdaniec, E. Dziemidowicz-Borys, A. Kurland, P. M. Deya, D. Quinonero, C. Garau, Eur. J. Org. Chem. 2005, 179–183.
- 12
- 12aT. Okubo, S. Kitagawa, M. Kondo, H. Matsuzaka, T. Ishii, Angew. Chem. 1999, 111, 980–983;
Angew. Chem. Int. Ed. 1999, 38, 931–933;
10.1002/(SICI)1521-3773(19990401)38:7<931::AID-ANIE931>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 12bS. Furukawa, T. Okubo, S. Masaoka, D. Tanaka, H. C. Chang, S. Kitagawa, Angew. Chem. 2005, 117, 2760–2764; Angew. Chem. Int. Ed. 2005, 44, 2700–2704.
- 13S. Masaoka, G. Akiyama, S. Horike, S. Kitagawa, T. Ida, K. Endo, J. Am. Chem. Soc. 2003, 125, 1152–1153.
- 14This nucleophilic displacement reaction is characteristic of N-heterocyclic chelate ligands such as 2,2′-bipyridyl (bpy) and 1,10-phenanthroline (phen). Coordination of N-heterocyclic chelate ligands to a metal ion activates the α carbon atoms of the ligands towards nucleophilic attack. For details of this type of reaction, see:
- 14aR. D. Gillard, Coord. Chem. Rev. 1975, 16, 67–94;
- 14bX. M. Zhang, M. L. Tong, X. M. Chen, Angew. Chem. 2002, 114, 1071–1073;
Angew. Chem. Int. Ed. 2002, 41, 1029–1031.
10.1002/1521-3773(20020315)41:6<1029::AID-ANIE1029>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 15A. F. Wells, Three-Dimensional Nets and Polyhedra, Wiley, New York, 1977.
- 16This distance is slightly shorter than the sum of van der Waals radii. The van der Waals radii of carbon, nitrogen, and oxygen are 1.7, 1.5 and 1.5 Å, respectively.
- 17The XRPD patterns of heated 1⊃THF show that the Bragg peaks become broad and disappear with elongation of the a and b axes and shrinking along the c axis in the temperature range 30–80 °C. These observations and the results of TGA demonstrate that the guest molecules play an important role in stabilizing the crystal structures.
- 18I. Alkorta, I. Rozas, M. L. Jimeno, J. Elguero, Struct. Chem. 2001, 12, 459–464.
- 19J. T. Rademacher, K. Kanakarajan, A. W. Czarnik, Synthesis 1994, 378–380.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.