A Formal Total Synthesis of (+)-Pinnatoxin A†
Satoshi Sakamoto Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorHayato Sakazaki
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorKoji Hagiwara
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorKei Kamada
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorKento Ishii
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorTakeshi Noda Prof. Dr.
Department of Applied Chemistry, Faculty of Engineering, Kanagawa Institute of Technology, Atsugi 243-0292,, Japan
Search for more papers by this authorMasayuki Inoue Prof. Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Research and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan
Search for more papers by this authorMasahiro Hirama Prof. Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorSatoshi Sakamoto Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorHayato Sakazaki
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorKoji Hagiwara
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorKei Kamada
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorKento Ishii
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorTakeshi Noda Prof. Dr.
Department of Applied Chemistry, Faculty of Engineering, Kanagawa Institute of Technology, Atsugi 243-0292,, Japan
Search for more papers by this authorMasayuki Inoue Prof. Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Research and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan
Search for more papers by this authorMasahiro Hirama Prof. Dr.
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan, Fax: (+81) 22-217-6566
Search for more papers by this authorThis work was supported by CREST, Japan Science and Technology Agency (JST). A fellowship to H.S. from the Japan Society for the Promotion of Science (JSPS) is gratefully acknowledged. We thank Professor Y. Kishi (Harvard University) for providing NMR spectra of synthetic intermediates, Professor D. Uemura (Nagoya University) for providing natural pinnatoxin A, and Professor K. Nagasawa (Tokyo University of Agriculture and Technology) for helpful discussions.
Graphical Abstract
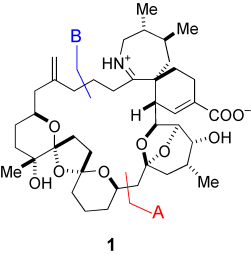
Die leistungsstarke Dithiankupplung (A) zweier Fragmente und die anschließende Makrocyclisierung durch Ringschlussmetathese (B) lieferten den vollständigen 27-gliedrigen Carbocyclus von (+)-Pinnatoxin A (1), einem wesentlichen toxischen Prinzip, das für das Auftreten von Schalentiervergiftungen in China und Japan verantwortlich ist.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z461802_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. Uemura, T. Chou, T. Haino, A. Nagatsu, S. Fukuzawa, S. Zheng, H. Chen, J. Am. Chem. Soc. 1995, 117, 1155–1156;
- 1bT. Chou, O. Kamo, D. Uemura, Tetrahedron Lett. 1996, 37, 4023–4026.
- 2For the structural determination of related molecules, see: pinnatoxin B,C:
- 2aN. Takada, N. Umemura, K. Suenaga, T. Chou, A. Nagatsu, T. Haino, K. Yamada, D. Uemura, Tetrahedron Lett. 2001, 42, 3491–3494; pinnatoxin D:
- 2bT. Chou, T. Haino, M. Kuramoto, D. Uemura, Tetrahedron Lett. 1996, 37, 4027–4030; spirolides:
- 2cT. Hu, J. M. Curtis, Y. Oshima, M. A. Quilliam, J. A. Walter, W. M. Watson-Wright, J. L. C. Wright, J. Chem. Soc. Chem. Commun. 1995, 2159–2161;
- 2dM. Falk, I. W. Burton, T. Hu, J. A. Walter, J. L. C. Wright, Tetrahedron 2001, 57, 8659–8665; pteriatoxins:
- 2eN. Takada, N. Umemura, K. Suenaga, D. Uemura, Tetrahedron Lett. 2001, 42, 3495–3497.
- 3S. Z. Zheng, F. L. Huang, S. C. Chen, X. F. Tan, J. B. Zuo, J. Peng, R. W. Xie, Chin. J. Mar. Drugs 1990, 33, 33–35.
- 4For synthetic studies of 1 from this laboratory, see:
- 4aT. Noda, A. Ishiwata, S. Uemura, S. Sakamoto, M. Hirama, Synlett 1998, 298–300;
- 4bA. Ishiwata, S. Sakamoto, T. Noda, M. Hirama, Synlett 1999, 692–694;
- 4cA. Nitta, A. Ishiwata, T. Noda, M. Hirama, Synlett 1999, 695–696;
- 4dJ. Wang, S. Sakamoto, K. Kamada, A. Nitta, T. Noda, H. Oguri, M. Hirama, Synlett 2003, 891–893.
- 5For synthetic studies of 1 from other laboratories, see:
- 5aT. Sugimoto, J. Ishihara, A. Murai, Tetrahedron Lett. 1997, 38, 7379–7382;
- 5bJ. Ishihara, T. Sugimoto, A. Murai, Synlett 1998, 603–606;
- 5cB. D. Suthers, M. F. Jacobs, W. Kitching, Tetrahedron Lett. 1998, 39, 2621–2624;
- 5dT. Sugimoto, J. Ishihara, A. Murai, Synlett 1999, 541–544;
- 5eJ. Ishihara, S. Tojo, A. Kamikawa, A. Murai, Chem. Commun. 2001, 1392–1393;
- 5fS. Nakamura, J. Inagaki, T. Sugimoto, M. Kudo, M. Nakajima, S. Hashimoto, Org. Lett. 2001, 3, 4075–4078;
- 5gS. Nakamura, J. Inagaki, M. Kudo, T. Sugimoto, K. Obara, M. Nakajima, S. Hashimoto, Tetrahedron 2002, 58, 10 353–10 374;
- 5hS. Nakamura, J. Inagaki, T. Sugimoto, Y. Ura, S. Hashimoto, Tetrahedron 2002, 58, 10 375–10 386.
- 6For synthetic studies of spirolides, see:
- 6aD. P. Furkert, M. A. Brimble, Org. Lett. 2002, 4, 3655–3658;
- 6bM. Trzoss, M. A. Brimble, Synlett 2003, 2042–2046, and references therein.
- 7
- 7aJ. A. McCauley, K. Nagasawa, P. A. Lander, S. G. Mischke, M. A. Semones, Y. Kishi, J. Am. Chem. Soc. 1998, 120, 7647–7648;
- 7bK. Nagasawa, J. Synth. Org. Chem. Jpn. 2000, 58, 877–886.
- 8For reviews, see:
- 8aA. Fürstner, Angew. Chem. 2000, 112, 3140–3172;
10.1002/1521-3757(20000901)112:17<3140::AID-ANGE3140>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3013–3043;
- 8bT. M. Trnka, R. H. Grubbs, Acc. Chem. Res. 2001, 34, 18–29;
- 8c Handbook of Metathesis, Vol. 2 (Ed.: ), Wiley-VCH, Weinheim, 2003.
- 9
- 9aG. Stork, L. D. Cama, D. R. Coulson, J. Am. Chem. Soc. 1974, 96, 5268–5270;
- 9bG. Stork, J. F. Cohen, J. Am. Chem. Soc. 1974, 96, 5270–5272; for a review, see:
- 9cF. F. Fleming, B. C. Shook, Tetrahedron 2002, 58, 1–23.
- 10
- 10aY. Gao, R. M. Hanson, J. M. Klunder, S. Y. Ko, H. Masamune, K. B. Sharpless, J. Am. Chem. Soc. 1987, 109, 5765–5780;
- 10bT. Katsuki, V. S. Martin, Org. React. 1996, 48, 1–299.
- 11J. M. Finan, Y. Kishi, Tetrahedron Lett. 1982, 23, 2719–2722.
- 12
- 12aG. A. Crispino, K. S. Jeong, H. C. Kolb, Z. M. Wang, D. Xu, K. B. Sharpless, J. Org. Chem. 1993, 58, 3785–3786;
- 12bP. G. Andersson, K. B. Sharpless, J. Am. Chem. Soc. 1993, 115, 7047–7048; for a review, see:
- 12b2H. C. Kolb, M. S. VanNieuwenhze, K. B. Sharpless, Chem. Rev. 1994, 94, 2483–2547.
- 13For a recent review on synthesis of bis-spiroacetals, see: M. A. Brimble, F. F. Farès, Tetrahedron 1999, 55, 7661–7706.
- 14Existence of this intramolecular hydrogen bond was suggested from the X-ray analysis of a closely related substrate with free hydroxy groups at C10 and C24.[4a] Furthermore, acidic treatment of a 10-OH-protected substrate resulted in nonselective formation of the desired spiroacetal and its C19-epimer despite the seemingly favorable anomeric effect at C19 of the desired compound.
- 15P. L. Barili, G. Berti, G. Catelani, C. Cini, F. D′Andrea, E. Mastrorilli, Carbohydr. Res. 1995, 278, 43–57.
- 16
- 16aH. J. Bestmann, Angew. Chem. 1965, 77, 651–666; Angew. Chem. Int. Ed. Engl. 1965, 4, 645–660;
- 16bR. S. Mali, S. G. Tilve, S. N. Yeola, A. R. Manekar, Heterocycles 1987, 26, 121–127.
- 17A. P. Kozikowski, J.-P. Wu, Tetrahedron Lett. 1987, 28, 5125–5128.
- 18H. C. Brown, R. Liotta, C. G. Scouten, J. Am. Chem. Soc. 1976, 98, 5297–5301.
- 19The intermediacy of epoxide 4 was supported by its isolation without the subsequent addition of KN(TMS)2.
- 20The stereochemistry of C5 and C31 were determined by X-ray crystallographic analysis of an analogue derivatized from 29; see Supporting Information for detail.
- 21F. N. Tebbe, G. W. Parshall, G. S. Reddy, J. Am. Chem. Soc. 1978, 100, 3611–3613.
- 22W. C. Still, J. C. Barrish, J. Am. Chem. Soc. 1983, 105, 2487–2489.
- 23
- 23aD. R. Williams, S.-Y. Sit, J. Am. Chem. Soc. 1984, 106, 2949–2954;
- 23bA. B. Smith III, S. M. Condon, J. A. McCauley, J. L. Leazer, Jr.,J. W. Leahy, R. E. Maleczka, Jr., J. Am. Chem. Soc. 1997, 119, 947–961;
- 23cA. B. Smith III, S. M. Condon, J. A. McCauley, Acc. Chem. Res. 1998, 31, 35–46.
- 24M. Scholl, S. Ding, C. W. Lee, R. H. Grubbs, Org. Lett. 1999, 1, 953–956.
- 25G. Stork, K. Zhao, Tetrahedron Lett. 1989, 30, 287–290.
- 26While initial treatment with TFA mainly removed the TMS and TES groups, the TIPS and acetonide groups were deprotected by subjection to CSA/MeOH. This stepwise deprotection scheme appears to be superior to a single-step approach in terms of yield.
- 27
- 27aH.-D. Becker, A. Björk, E. Adler, J. Org. Chem. 1980, 45, 1596–1600;
- 27bE. J. Corey, C. Shih, N.-Y. Shih, K. Shimoji, Tetrahedron Lett. 1984, 25, 5013–5016.
- 28W. S. Mahoney, D. M. Brestensky, J. M. Stryker, J. Am. Chem. Soc. 1988, 110, 291–293.
- 29D. B. Dess, J. C. Martin, J. Am. Chem. Soc. 1991, 113, 7277–7287.
- 30T. Aoyama, N. Sonoda, M. Yamauchi, K. Toriyama, M. Anzai, A. Ando, T. Shioiri, Synlett 1998, 35–36.
- 31L. J. Mathias, Synthesis 1979, 561–576.
- 32For related examples, see:
- 32aB. M. Trost, S. R. Pulley, Tetrahedron Lett. 1995, 36, 8737–8740;
- 32bJ. Zhu, D. Ma, Angew. Chem. 2003, 115, 5506–5509; Angew. Chem. Int. Ed. 2003, 42, 5348–5351.
- 33Kishi reported that heating 57 at 200 °C under vacuum and treating the resultant imine with TFA afforded pinnatoxin A (1).[7]
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.