A Stereodivergent Synthesis of Virantmycin by an Enzyme-Mediated Diester Desymmetrization and a Highly Hindered Aryl Amination†
Thomas G. Back Prof. Dr.
Department of Chemistry, University of Calgary, Calgary, AB, T2N 1N4, Canada, Fax: (+1) 403-289-9488
Search for more papers by this authorJeremy E. Wulff
Department of Chemistry, University of Calgary, Calgary, AB, T2N 1N4, Canada, Fax: (+1) 403-289-9488
Search for more papers by this authorThomas G. Back Prof. Dr.
Department of Chemistry, University of Calgary, Calgary, AB, T2N 1N4, Canada, Fax: (+1) 403-289-9488
Search for more papers by this authorJeremy E. Wulff
Department of Chemistry, University of Calgary, Calgary, AB, T2N 1N4, Canada, Fax: (+1) 403-289-9488
Search for more papers by this authorWe thank the Natural Sciences and Engineering Research Council of Canada (NSERC) for financial support. J.E.W. thanks, the NSERC, the Alberta Heritage Foundation for Medical Research, and the Izaak Walton Killam Foundation for Postgraduate Scholarships. We thank Dr. B. A. Keay for helpful discussions and a generous gift of BINAPFu.
Graphical Abstract
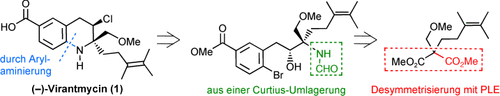
Beide Enantiomere des Antivirusmittels Virantmycin wurden in einer Reaktionsfolge erhalten, deren Schlüsselschritte eine hoch stereoselektive, durch Schweineleber-Esterase (PLE) vermittelte Desymmetrisierung eines prochiralen Diesters und eine bemerkenswert effektive intramolekulare Arylaminierung eines ortho-substituierten Arylbromids mit einem stark gehinderten α-quartären Formamid sind (siehe Retrosyntheseschema).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z461327_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Ōmura, A. Nakagawa, H. Hashimoto, R. Oiwa, Y. Iwai, A. Hirano, N. Shibukawa, Y. Kojima, J. Antibiot. 1980, 33, 1395.
- 2A. Nakagawa, Y. Iwai, H. Hashimoto, N. Miyazaki, R. Oiwa, Y. Takahashi, A. Hirano, N. Shibukawa, Y. Kojima, S. Ōmura, J. Antibiot. 1981, 34, 1408.
- 3Y. Morimoto, K. Oda, H. Shirahama, T. Matsumoto, S. Ōmura, Chem. Lett. 1988, 909.
- 4C. M. Pearce, J. K. M. Sanders, J. Chem. Soc. Perkin Trans. 1 1990, 409.
- 5Y. Morimoto, F. Matsuda, H. Shirahama, Synlett 1991, 202.
- 6
- 6aM. L. Hill, R. A. Raphael, Tetrahedron Lett. 1986, 27, 1293;
- 6bM. L. Hill, R. A. Raphael, Tetrahedron 1990, 46, 4587.
- 7Y. Morimoto, F. Matsuda, H. Shirahama, Tetrahedron 1996, 52, 10 609.
- 8H. Steinhagen, E. J. Corey, Org. Lett. 1999, 1, 823.
- 9Y. Morimoto, H. Shirahama, Tetrahedron 1996, 52, 10 631.
- 10M. Ori, N. Toda, K. Takami, K. Tago, H. Kogen, Angew. Chem. 2003, 115, 2644;
10.1002/ange.200351069 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 2540.
- 11
- 11aT. G. Back, R. J. Bethell, M. Parvez, D. Wehrli, J. Org. Chem. 1998, 63, 7908;
- 11bA. N. De Silva, C. L. Francis, A. D. Ward, Aust. J. Chem. 1993, 46, 1657.
- 12
- 12aFor a general review, see: H. G. Davies, R. H. Green, D. R. Kelly, S. M. Roberts, Biotransformations in Preparative Organic Chemistry, Academic Press, London, 1989, chap. 2;
- 12bF. Björkling, J. Boutelje, S. Gatenbeck, K. Hult, T. Norin, P. Szmulik, Tetrahedron 1985, 41, 1347;
- 12cE. J. Toone, M. J. Werth, J. B. Jones, J. Am. Chem. Soc. 1990, 112, 4946.
- 13The acyl fluorides were prepared by the general method of: S. Groβ, S. Laabs, A. Scherrmann, A. Sudau, N. Zhang, U. Nubbemeyer, J. Prakt. Chem. 2000, 342, 711.
- 14Diester 6 was prepared by treatment of methyl 4-bromo-3-bromomethylbenzoate with NaCN, followed by alkaline hydrolysis of the corresponding benzyl nitrile and Fischer esterification with methanol.
- 15A. P. Krapcho, A. J. Lovey, Tetrahedron Lett. 1973, 957.
- 16T. Shioiri, K. Ninomiya, S. Yamada, J. Am. Chem. Soc. 1972, 94, 6203.
- 17For selected reviews, see:
- 17aJ. P. Wolfe, S. Wagaw, J.-F. Marcoux, S. L. Buchwald, Acc. Chem. Res. 1998, 31, 805;
- 17bJ. F. Hartwig, Acc. Chem. Res. 1998, 31, 852;
- 17cJ. F. Hartwig in Handbook of Organopalladium Chemistry for Organic Synthesis, Vol. 1 (Ed.: ), Wiley, Hoboken, NJ, 2002, pp. 1051–1096.
10.1002/0471212466.ch42 Google Scholar
- 18
- 18aN. G. Andersen, M. Parvez, R. McDonald, B. A. Keay, Can. J. Chem. 2004, 82, 145;
- 18bfor a review of 2-furylphosphine ligands in transition-metal-mediated reactions, see: N. G. Andersen, B. A. Keay, Chem. Rev. 2001, 101, 997.
- 19aBINAP=2,2′-bis(diphenylphosphanyl)-1,1′-binaphthyl: J. P. Wolfe, S. Wagaw, S. L. Buchwald, J. Am. Chem. Soc. 1996, 118, 7215;
- 19bDPPF=1,1′-bis(diphenylphosphanyl)ferrocene: M. S. Driver, J. F. Hartwig, J. Am. Chem. Soc. 1996, 118, 7217;
- 19cPCy3: N. P. Reddy, M. Tanaka, Tetrahedron Lett. 1997, 38, 4807;
- 19do-biphenylPCy2 and o-biphenylPtBu2: J. P. Wolfe, H. Tomori, J. P. Sadighi, J. Yin, S. L. Buchwald, J. Org. Chem. 2000, 65, 1158;
- 19eDPEphos=2,2′-bis(diphenylphosphanyl)-1,1′-diphenyl ether: J. P. Sadighi, M. C. Harris, S. L. Buchwald, Tetrahedron Lett. 1998, 39, 5327;
- 19fMAP=2′-dimethylamino-2-(diphenylphosphanyl)-1,1′-binaphthalene: S. Vyskočil, M. Smrčina, P. Kočovský, Tetrahedron Lett. 1998, 39, 9289;
- 19gIMES hydrochloride=1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene hydrochloride: G. A. Grasa, M. S. Viciu, J. Huang, S. P. Nolan, J. Org. Chem. 2001, 66, 7729.
- 20For an aryl amination with tert-butylamine, see:
- 20aH. Siebeneicher, I. Bytschkov, S. Doye, Angew. Chem. 2003, 115, 3151;
10.1002/ange.200351345 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3042;
- 20bI. Bytschkov, H. Siebeneicher, S. Doye, Eur. J. Org. Chem. 2003, 2888; for an aryl amination with N-tert-butylaniline, see:
- 20cM. Prashad, X. Y. Mak, Y. Liu, O. Repič, J. Org. Chem. 2003, 68, 1163.
- 21G. Mann, Q. Shelby, A. H. Roy, J. F. Hartwig, Organometallics 2003, 22, 2775.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.