Dramatic Enhancement of Catalytic Activity in an Ionic Liquid: Highly Practical Friedel–Crafts Alkenylation of Arenes with Alkynes Catalyzed by Metal Triflates†
Choong Eui Song Prof.
Institute of Basic Science, Department of Chemistry, Sungkyunkwan University, 300 Chunchun, Jangan, Suwon, Kyunggi, 440-746, Korea, Fax: (+82) 31-290-7075
Search for more papers by this authorDa-un Jung
Life Sciences Division, Korea Institute of Science and Technology, PO Box 131, Cheongryang, Seoul 130-650, Korea, Fax: (+82) 2-958-5189
Search for more papers by this authorSu Yhen Choung
Institute of Basic Science, Department of Chemistry, Sungkyunkwan University, 300 Chunchun, Jangan, Suwon, Kyunggi, 440-746, Korea, Fax: (+82) 31-290-7075
Search for more papers by this authorEun Joo Roh Dr.
Life Sciences Division, Korea Institute of Science and Technology, PO Box 131, Cheongryang, Seoul 130-650, Korea, Fax: (+82) 2-958-5189
Search for more papers by this authorSang-gi Lee Dr.
Life Sciences Division, Korea Institute of Science and Technology, PO Box 131, Cheongryang, Seoul 130-650, Korea, Fax: (+82) 2-958-5189
Search for more papers by this authorChoong Eui Song Prof.
Institute of Basic Science, Department of Chemistry, Sungkyunkwan University, 300 Chunchun, Jangan, Suwon, Kyunggi, 440-746, Korea, Fax: (+82) 31-290-7075
Search for more papers by this authorDa-un Jung
Life Sciences Division, Korea Institute of Science and Technology, PO Box 131, Cheongryang, Seoul 130-650, Korea, Fax: (+82) 2-958-5189
Search for more papers by this authorSu Yhen Choung
Institute of Basic Science, Department of Chemistry, Sungkyunkwan University, 300 Chunchun, Jangan, Suwon, Kyunggi, 440-746, Korea, Fax: (+82) 31-290-7075
Search for more papers by this authorEun Joo Roh Dr.
Life Sciences Division, Korea Institute of Science and Technology, PO Box 131, Cheongryang, Seoul 130-650, Korea, Fax: (+82) 2-958-5189
Search for more papers by this authorSang-gi Lee Dr.
Life Sciences Division, Korea Institute of Science and Technology, PO Box 131, Cheongryang, Seoul 130-650, Korea, Fax: (+82) 2-958-5189
Search for more papers by this authorThis work was supported by the BK21 project of the Ministry of Education in Sungkyunkwan University (SKKU) and the National Research Laboratory Program in Korea Institute of Science and Technology (KIST).
Graphical Abstract
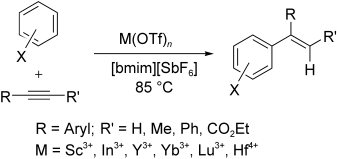
Eine einfache und hoch effiziente Variante der Friedel-Crafts-Alkenylierung von Arenen beruht auf dem Einsatz eines Metalltriflat-Katalysators in einer ionischen Flüssigkeit (siehe Schema, bmim=1-Butyl-3-methylimidazolium, Tf=Trifluormethansulfonyl). So wird die katalytische Aktivität deutlich erhöht und die Bildung von Nebenprodukten zurückgedrängt. Mit diesem System gelangen einige Reaktionen, die in gängigen organischen Lösungsmitteln nicht möglich sind.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z460292_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews of Friedel–Crafts alkylation reactions, see:
- 1a Friedel–Crafts and Related Reactions, Vol. II, part 1 (Ed.: ), Wiley-Interscience, New York, 1964;
- 1b Friedel–Crafts Alkylation Chemistry. A Centry of Discovery (Eds.: ), Marcel Dekker, New York, 1984;
- 1cG. A. Olah, R. Krishnamurit, G. K. S. Prakash, in Friedel–Crafts Alkylations in Comprehensive Organic Synthesis (Eds.: ), Pergamon, Oxford, 1991.
- 2O. W. Cook, V. J. Chaber, J. Am. Chem. Soc. 1921, 43, 334; J. S. Reichert, J. A. Nieuwland, J. Am. Chem. Soc. 1923, 45, 3090; J. A. Reilly, J. A. Nieuwland, J. Am. Chem. Soc. 1928, 50, 2564.
- 3T. Tsuchimoto, T. Maeda, E. Shirakawa, Y. Kawakami, Chem. Commun. 2000, 1573.
- 4G. Sartori, F. Bigi, A. Pastorio, C. Porta, A. Arienti, R. Maggi, N. Moretti, G. Gnappi, Tetrahedron Lett. 1995, 36, 9177.
- 5G. Casiraghi, G. Casnati, G. Puglia, G. Sartori, G. Terenghi, Synthesis 1977, 122.
- 6M. Yamaguchi, Y. Kido, A. Hayashi, M. Hirama, Angew. Chem. 1997, 109, 1370;
10.1002/ange.19971091219 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1313; Y. Kido, S. Yoshimura, M. Yamaguchi, T. Uchimaru, Bull. Chem. Soc. Jpn. 1999, 72, 1445.
- 7M. Yamaguch, A. Hayashi, M. Hirama, J. Am. Chem. Soc. 1995, 117, 1151.
- 8For recent reviews on ionic liquids, see:
- 8aC. E. Song, Chem. Commun. 2004, 1033;
- 8bR. Sheldon, Chem. Commun. 2001, 2399;
- 8cP. Wasserscheid, W. Kein, Angew. Chem. 2000, 112, 3926;
10.1002/1521-3757(20001103)112:21<3926::AID-ANGE3926>3.0.CO;2-U Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3772;10.1002/1521-3773(20001103)39:21<3772::AID-ANIE3772>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 8dT. Welton, Chem. Rev. 1999, 99, 2071.
- 9Our recent examples:
- 9aC. E. Song, W. H. Shim, E. J. Roh, J. H. Choi, Chem. Commun. 2000, 1695;
- 9bC. E. Song, E. J. Roh, Chem. Commun. 2000, 837;
- 9cC. E. Song, W. H. Shim, E. J. Roh, S.-g. Lee, J. H. Choi, Chem. Commun. 2001, 1122;
- 9dS.-g. Lee, J. W. Park, J. Kang, J. K. Lee, Chem. Commun. 2001, 1698;
- 9eD. W. Kim, C. E. Song, D. Y. Chi, J. Am. Chem. Soc. 2002, 124, 10 278;
- 9fS.-g. Lee, J. W. Park, Bull. Korean Chem. Soc. 2002, 23, 1367;
- 9gD. W. Kim, C. E. Song, D. Y. Chi, J. Org. Chem. 2003, 68, 4281;
- 9hE. J. Kim, S. Y. Ko, C. E. Song, Helv. Chim. Acta 2003, 86, 894;
- 9iS.-g. Lee, Y. J. Zhang, P. Z. Yu, H. Yoon, C. E. Song, J. H. Choi, J. Hong, Chem. Commun. 2003, 2624;
- 9jS.-g. Lee, J. W. Park, J. Mol. Catal. A 2003, 194, 49.
- 10In sharp contrast to these results, only very small amounts of the alkenylated product was obtained (<2 % yield) in the hydrophilic ionic liquids, [bmim][BF4] or [bmim][OTf].
- 11Recently, Rogers and co-workers reported the instability of the ionic liquids’ counterion PF6 towards moisture, which resulted in hydrolysis and the formation of HF (R. P. Swatloski, J. D. Holbrey, R. D. Rogers, Green Chem. 2003, 5, 361). Therefore, to examine the possible catalytic effect of HF on this reaction, we also carried out the reaction only in [bmim][SbF6] or in a mixture of [bmim][SbF6] and water (1:1, v/v) without any metal triflate catalyst. However, the reaction did not occur at all.
- 12A new synthetic route to alkenylated arenes involving Pd- and Pt-catalyzed addition of arenes to alkynes in the presence of trifluoroacetic acid was recently reported:
- 12aC. Jia, D. Piao, J. Oyamada, W. Lu, T. Kitamura, Y. Fujiwara, Science 2000, 287, 1992;
- 12bC. Jia, W. Lu, J. Oyamada, T. Kitamura, K. Matsuda, M. Irie, Y. Fujiwara, J. Am. Chem. Soc. 2000, 122, 7252.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.