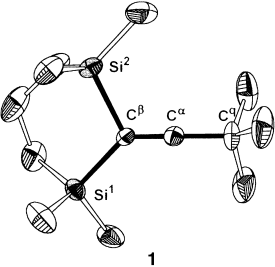
The X-ray Structure of a Vinyl Cation†
Thomas Müller Dr.
Institut für Anorganische und Analytische Chemie, der Johann Wolfgang Goethe–Universität Frankfurt/Main, Marie Curie Strasse 11, 60439 Frankfurt/Main, Germany, Fax: (+49) 697-982-9188
Search for more papers by this authorMark Juhasz
Department of Chemistry, University of California, Riverside 92521-0403, USA, Fax: (+1) 909-787-2027
Search for more papers by this authorChristopher A. Reed Prof. Dr.
Department of Chemistry, University of California, Riverside 92521-0403, USA, Fax: (+1) 909-787-2027
Search for more papers by this authorThomas Müller Dr.
Institut für Anorganische und Analytische Chemie, der Johann Wolfgang Goethe–Universität Frankfurt/Main, Marie Curie Strasse 11, 60439 Frankfurt/Main, Germany, Fax: (+49) 697-982-9188
Search for more papers by this authorMark Juhasz
Department of Chemistry, University of California, Riverside 92521-0403, USA, Fax: (+1) 909-787-2027
Search for more papers by this authorChristopher A. Reed Prof. Dr.
Department of Chemistry, University of California, Riverside 92521-0403, USA, Fax: (+1) 909-787-2027
Search for more papers by this authorWe thank Dr. Fook Tham for solution of the X-ray crystal structure. This work was supported by NSF grant CHE-0095206 and by the German Israeli Foundation (GIF).
Graphical Abstract
Erfolgreich kristallisiert: Thermodynamische Stabilisierung durch zwei β-Silylsubstituenten und im Wesentlichen nichtnucleophile Reaktionsbedingungen waren die Voraussetzungen für die Herstellung des ersten strukturell gut charakterisierten Vinylkations 1 (siehe Bild). Das Auftreten von β-Si-C-Hyperkonjugation in 1 wird durch ungewöhnlich lange Si-Cβ-Bindungen angezeigt.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z52986_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For a recent monograph on vinyl cations: Dicoordinated Carbocations (Eds.: ), Wiley, New York, 1997.
- 2
- 2aC. A. Grob, J. Csapilla, G. Cseh, Helv. Chim. Acta 1964, 47, 1590;
- 2bC. A. Grob in Dicoordinated Carbocations (Eds.: ), Wiley, 1997, chap. 1, p. 1.
- 3
- 3aM. Hanack, Acc. Chem. Res. 1976, 9, 364;
- 3bT. Kitamura, H. Taniguchi, Y. Tsuno in Dicoordinated Carbocations (Eds.: ), Wiley, New York, 1997, chap. 7, p. 321;
- 3cL. R. Subramanian, M. Hanack, Chem. Rev. 1972, 72, 1465.
- 4For reviews see:
- 4aV. Lucchini, G. Modena, L. Pasquato in Dicoordinated Carbocations (Eds.: ), Wiley, New York, 1997, chap. 6, p. 237.
- 5H.-U. Siehl in Dicoordinated Carbocations (Eds.: ), Wiley, 1997, chap. 5, p. 189.
- 6For a previous report on the isolation of the neutral solid metallacarborane [CpFeIIHCB9H9CCCH2] containing a -CCH2 group, see. L. I. Zakharkin, V. V. Kobak, J. Organomet. Chem. 1984, 270, 229.
- 7The isoelectronic neutral methylene boranes (R3Si)2CBR were previously synthesized and structurally characterized.
- 7aR. Boese, P. Paetzold, A. Tapper, R. Ziembinski, Chem. Ber. 1989, 122, 1057;
- 7bM. Pilz, M. Stadler, R. Hunold, J. Allwohn, W. Massa, A. Berndt, Angew. Chem. 1989, 101, 761; Angew. Chem. Int. Ed. Engl. 1989, 28, 784.
- 8T. Müller, R. Meyer, D. Lennartz, H.-U. Siehl, Angew. Chem. 2000, 112, 3203;
Angew. Chem. Int. Ed. 2000, 39, 3074.
10.1002/1521-3773(20000901)39:17<3074::AID-ANIE3074>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 9C. A. Reed, Acc. Chem. Res. 1998, 31, 133.
- 10Y. Takeuchi, T. Takayama in The chemistry of organosilicon compounds, Vol. 2 (Eds.: ), Wiley, New York, 1998 chap. 6, p. 267.
- 11A small 1J(SnC) coupling constant was detected in a related distannylmethylene borane. M. Pilz, H. Michel, A. Berndt, Angew. Chem. 1990, 102, 438; Angew. Chem. Int. Ed. Engl. 1990, 29, 409.
- 12Crystal data for 1 [CB11H6Br6], C14H33B11Br6Si2, Mr=860.69, crystal dimensions 0.19×0.18×0.04 mm3, monoclinic, space group P2(1)/c, a=12.250(3), b=13.034(3), c=20.639(5) Å, β=104.293(6)°, V=3193.3(13) Å3, Z=4, ρcalcd*=1.790 Mg m−3, μ(MoKα)=7.701 mm−1, T=223(2) K, F(000)=1648, 2θmax=49.42°. 17 628 reflections measured, of which 5441 were unique (Rint=0.0551). Final R1=0.0481 with wR2=0.1165 for 5441 observed reflections with I>2σ(I). * Note: The bromo-carborane was over brominated by about 6 %. The density was calculated by using the site occupancy factor ratio of 6 %/94 % for Br7/H6 attached to B6 atom. The slight over bromination of the carborane is figured into the X-ray model and solution, thus improving the accuracy and R value. CCDC-220463 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 13
- 13aR. Sustmann, J. E. Williams, M. J. S. Dewar, L. C. Allen, P. von R. Schleyer, J. Am. Chem. Soc. 1969, 91, 5350.
- 13bFor a review: Y. Apeloig, T. Müller in Dicoordinated Carbocations (Eds.: ), Wiley, New York, 1997, chap. 2, p. 9.
- 14M. Kaftory, M. Kapon, M. Botoshansky in The chemistry of organosilicon compounds, Vol. 2 (Eds.: ), Wiley, New York, 1998, chap. 5, p. 181.
- 15All calculations were performed with Gaussian 98 (Revision A.7), M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian, Inc., Pittsburgh, PA, 1998 and Gaussian 03 (Revision B.03), M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian, Inc. Pittsburgh, PA, 2003.
- 16
- 16aA. D. Becke, Phys. Rev. A 1988, 38, 3098;
- 16bA. D. Becke, J. Chem. Phys. 1993, 98, 5648;
- 16cB. G. Johnson, P. M. W. Gill, J. A. Pople, J. Chem. Phys. 1993, 98, 5612.
- 17According to density functional calculations at the B3LYP/6-31G(d) level of theory β-disilyl substituted vinyl cations having π-donating substituents at Cα do not show this unsymmetrical arrangement of the β-silylgroups. Thus, for the analogous phenyl substituted vinyl cation, equal SiCβ bond lengths of 197.3 pm have been calculated.[8]
- 18The calculated frequency of
 =2031 cm−1 is scaled with the global scaling factor of 0.963 suggested by Rauhut and Pulay. G. Rauhut, P. Pulay, J. Phys. Chem. 1995, 99, 3093.
=2031 cm−1 is scaled with the global scaling factor of 0.963 suggested by Rauhut and Pulay. G. Rauhut, P. Pulay, J. Phys. Chem. 1995, 99, 3093.
- 19
- 19aR. Ditchfield, Mol. Phys. 1974, 27, 789;
- 19bK. Wolinski, J. F. Hilton, P. Pulay, J. Am. Chem. Soc. 1982, 104, 5667;
- 19cJ. Cheeseman, G. W. Trucks, T. A. Keith, M. Frisch, J. Chem. Phys. 1996, 104, 5497.
- 20
- 20aT. Helgaker, M. Watson, N. C. Handy, J. Chem. Phys. 2000, 113, 9402;
- 20bV. Sychrovsky, J. Gräfenstein, D. Cremer, J. Chem. Phys. 2000, 113, 3530.
- 21Mean value from δ29(Si1)=23.7 ppm and δ29(Si2)=39.9 ppm.
- 22DFT-based Gauge Independent Atomic Orbitals (GIAO) calculations fail to predict reliably δ13C NMR chemical shift for the CC+ unit in vinyl cations. For reliable predictions of the δ13C(Cα), the coupled-cluster method must be applied, which is, however, for 1 technically not feasible.
- 22aJ. F. Stanton, J. Gauss, H.-U. Siehl, Chem. Phys. Lett. 1996, 262, 183;
- 22bH.-U. Siehl, T. Müller, H.-U. Siehl, J. Phys. Org. Chem. 2003, 16, 577.
- 23δ13Ctheo=(1.049±0.06) δ13Cexp + (3.50±0.53); R=0.99991, 7 data points, for details see Supporting Information.
- 24Mean value from 1J(Si1Cβ)=17.2 Hz and 1J(Si2Cβ)=3.2 Hz.
- 25The Fermi contact contribution to 1J(Si1Cβ) is +18.0 Hz, compared to +52.6 Hz for 1J(Si1C1).
- 26
- 26aNBO 4.0. E. D. Glendening; J. K. Badenhoop, A. E. Reed, J. E. Carpenter, F. Weinhold, Theoretical Chemistry Institute, University of Wisconsin, Madison, WI, 1996;
- 26bA. E. Reed, L. A. Curtiss, F. Weinhold, Chem. Rev. 1988, 88, 899.
- 27Supporting Information (experimental details, analytical data, NMR spectra, absolute energies and structures of 1 at several theoretical levels, correlation between δ13Ctheo versus δ13Cexp) is available.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.