A Red, Green, and Blue (RGB) Polymeric Electrochromic Device (PECD): The Dawning of the PECD Era†
Gursel Sonmez Dr.
Department of Chemistry and Biochemistry and Exotic Materials Institute, University of California, Los Angeles, CA 90095-1569, USA, Fax: (+1) 310-8250767
Search for more papers by this authorClifton K. F. Shen Dr.
Department of Chemistry and Biochemistry and Exotic Materials Institute, University of California, Los Angeles, CA 90095-1569, USA, Fax: (+1) 310-8250767
Search for more papers by this authorYves Rubin Prof.
Department of Chemistry and Biochemistry and Exotic Materials Institute, University of California, Los Angeles, CA 90095-1569, USA, Fax: (+1) 310-8250767
Search for more papers by this authorFred Wudl Prof.
Department of Chemistry and Biochemistry and Exotic Materials Institute, University of California, Los Angeles, CA 90095-1569, USA, Fax: (+1) 310-8250767
Search for more papers by this authorGursel Sonmez Dr.
Department of Chemistry and Biochemistry and Exotic Materials Institute, University of California, Los Angeles, CA 90095-1569, USA, Fax: (+1) 310-8250767
Search for more papers by this authorClifton K. F. Shen Dr.
Department of Chemistry and Biochemistry and Exotic Materials Institute, University of California, Los Angeles, CA 90095-1569, USA, Fax: (+1) 310-8250767
Search for more papers by this authorYves Rubin Prof.
Department of Chemistry and Biochemistry and Exotic Materials Institute, University of California, Los Angeles, CA 90095-1569, USA, Fax: (+1) 310-8250767
Search for more papers by this authorFred Wudl Prof.
Department of Chemistry and Biochemistry and Exotic Materials Institute, University of California, Los Angeles, CA 90095-1569, USA, Fax: (+1) 310-8250767
Search for more papers by this authorWe gratefully acknowledge financial support from the Air Force Office of Scientific Research through F49620-00-1-0103 and the Army Research Office through MURI DAAD19-99-1-0316. Instrumentation for this research was partially funded by NSF grand DGE-0114443. The authors would like to thank Prof. John R. Reynolds at University of Florida for helpful discussions. We thank Dr. Hieu Duong and Ryan Chiechi for recording the video display of color switching.
Graphical Abstract
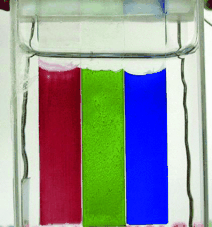
RGB-Farbraum komplettiert: Die elektrochemischen und optischen Eigenschaften des ersten elektrochemisch hergestellten leitfähigen Polymers, das in seiner Neutralform grün ist, werden beschrieben. Seine außerordentlich hohe Stabilität ist ein entscheidender Schritt vorwärts auf dem Gebiet elektrochromer Polymere, da dieses Polymer entsprechende rote und blaue leitfähige Polymere ergänzt (siehe Bild).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z52910_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Handbook of Conducting Polymers (Eds.: ), 2nd ed., Marcel Dekker, New York, 1998.
- 2H. J. Byker, (Gentex Corporation), 1990, US Patent No. 4902108.
- 3R. G. Mortimer, Chem. Soc. Rev. 1997, 26, 147.
- 4C. G. Granqvist, A. Azens, A. Hjelm, L. Kullman, G. A. Niklasson, D. Ronnow, M. Stromme Mattsson, M. Veszelei, G. Vaivars, Sol. Energy 1998, 63, 199.
- 5R. D. Rauh, Electrochim. Acta 1999, 44, 3165.
- 6J. J. M. Halls, C. A. Walsh, N. C. Greenham, E. A. Marseglia, R. H. Friend, S. C. Moratti, A. B. Holmes, Nature 1995, 376, 498.
- 7P. M. S. Monk, J. Electroanal. Chem. 1997, 432, 175.
- 8K. Bange, Sol. Energy Mater. Sol. Cells 1999, 58, 1.
- 9K. J. Albert, N. S. Lewis, C. L. Schauer, G. A. Sotzing, S. E. Stitzel, T. P. Vaid, D. R. Walt, Chem. Rev. 2000, 100, 2595.
- 10D. T. McQuade, A. E. Pullen, T. M. Swager, Chem. Rev. 2000, 100, 2537.
- 11R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. Dos Santos, J. L. Bredas, M. Logdlund, W. R. Salaneck, Nature 1999, 397, 121.
- 12A. Kraft, A. C. Grimsdale, A. B. Holmes, Angew. Chem. 1998, 110, 444;
10.1002/(SICI)1521-3757(19980216)110:4<416::AID-ANGE416>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 1998, 37, 402.10.1002/(SICI)1521-3773(19980302)37:4<402::AID-ANIE402>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 13C. J. Brabec, N. S. Sariciftci, J. C. Hummelen, Adv. Funct. Mater. 2001, 11, 15.
- 14P. K. H. Ho, D. S. Thomas, R. H. Friend, N. Tessler, Science 1999, 285, 233.
- 15I. Schwendeman, R. Hickman, G. Sonmez, P. Schottland, K. Zong, D. M. Welsh, J. R. Reynolds, Chem. Mater. 2002, 14, 3118.
- 16G. Sonmez, H. Meng, Q. Zhang, F. Wudl, Adv. Funct. Mater. 2003, 13, 726.
- 17G. Sonmez, H. Meng, F. Wudl, Chem. Mater., in press.
- 18G. Sonmez, I. Schwendeman, P. Schottland, K. Zong, J. R. Reynolds, Macromolecules 2003, 36, 639.
- 19B. C. Thompson, P. Schottland, G. Sonmez, J. R. Reynolds, Synth. Met. 2001, 119, 333.
- 20B. C. Thompson, P. Schottland, K. Zong, J. R. Reynolds, Chem. Mater. 2000, 12, 1563.
- 21A. M. McDonagh, S. R. Bayly, D. J. Riley, M. D. Ward, J. A. McCleverty, M. A. Cowin, C. N. Morgan, R. Varrazza, R. V. Penty, I. H. White, Chem. Mater. 2000, 12, 2523.
- 22G. Sonmez, P. Schottland, K. Zong, J. R. Reynolds, J. Mater. Chem. 2001, 11, 289.
- 23P. Chandrasekhar, G. C. Birur, P. Stevens, S. Rawal, E. A. Pierson, K. L. Miller, Synth. Met. 2001, 119, 293.
- 24I. Schwendeman, J. Hwang, D. M. Welsh, D. B. Tanner, J. R. Reynolds, Adv. Mater. 2001, 13, 634.
- 25H. Meng, D. Tucker, S. Chaffins, Y. Chen, R. Helgeson, B. Dunn, F. Wudl, Adv. Mater. 2003, 15, 146.
- 26W. C. Dautremont-Smith, Displays I, 1982, 3.
- 27P. M. S. Monk, R. J. Mortimer, D. R. Rosseinsky, Electrochromism: Fundamentals and Applications, VCH, Weinheim, 1995.
10.1002/9783527615377 Google Scholar
- 28K. Bange, T. Bambke, Adv. Mater. 1990, 2, 10.
- 29R. J. Mortimer, Electrochim. Acta 1999, 44, 2971.
- 30S. A. Sapp, G. A. Sotzing, J. R. Reynolds, Chem. Mater. 1998, 10, 2101.
- 31A. Kumar, D. M. Welsh, M. C. Morvant, F. Piroux, K. A. Abboud, J. R. Reynolds, Chem. Mater. 1998, 10, 896.
- 32J. Roncali, Chem. Rev. 1997, 97, 173.
- 33H. S. Nalwa, Handbook of Organic Conductive Molecules and Polymers, Wiley, New York, 1997.
- 34R. D. Overheim, D. L. Wagner, Light and Color, Wiley, New York, 1982.
- 35Since PDDTP was insoluble in any solvent, an alkyl-substituted counterpart of the monomer (Supporting Information; Figure S2) was synthesized and electrochemically polymerized under the same conditions. Then polymer films were reduced electrochemically in monomer-free electrolyte solution and dissolved in [D2]tetrachloroethane. 13C NMR spectroscopy of the resulting polymer demonstrated that hydrogen atoms on the α and α′ positions of the thiophene rings labeled with the letters c and d (Supporting Information) remained after electrochemical polymerization confirming the proposed structure for PDDTP. The related work for alkyl-substituted PDDTP will be published separately.
- 36A device made by using initials of “Exotic Materials Institute” is also available, Figure S8 of Supporting Information.
- 37R. T. Markus in Color for Science, Art and Technology (Ed.: ), Elsevier, Amsterdam, 1998, pp. 31–96.
- 38C. Kitamura, S. Tanaka, Y. Yamashita, Chem. Mater. 1996, 8, 570.
- 39Using a reported procedure (F. Babudri, V. Fiandanese, G. Marchese, A. Punzi, Tetrahedron Lett. 1995, 36, 7305), 3-thienyl Grignard reagent was prepared from the corresponding 3-bromothiophene.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.