Facile Syntheses of Copper(I) Alkynyl Clusters Stabilized by Hexafluoroacetylacetonate (hfac) Ligands: The Structure of [Cu26(hfac)11(1-pentynyl)15]†
Timothy C. Higgs Dr.
The Department of Chemistry The King's Buildings, The University of Edinburgh West Mains Road, Edinburgh, Lothian, Scotland, EH9 3JJ (UK) Fax: (+44) 131-650-6452
Search for more papers by this authorPhillip J. Bailey Dr.
The Department of Chemistry The King's Buildings, The University of Edinburgh West Mains Road, Edinburgh, Lothian, Scotland, EH9 3JJ (UK) Fax: (+44) 131-650-6452
Search for more papers by this authorSimon Parsons Dr.
The Department of Chemistry The King's Buildings, The University of Edinburgh West Mains Road, Edinburgh, Lothian, Scotland, EH9 3JJ (UK) Fax: (+44) 131-650-6452
Search for more papers by this authorPeter A. Tasker Prof.
The Department of Chemistry The King's Buildings, The University of Edinburgh West Mains Road, Edinburgh, Lothian, Scotland, EH9 3JJ (UK) Fax: (+44) 131-650-6452
Search for more papers by this authorTimothy C. Higgs Dr.
The Department of Chemistry The King's Buildings, The University of Edinburgh West Mains Road, Edinburgh, Lothian, Scotland, EH9 3JJ (UK) Fax: (+44) 131-650-6452
Search for more papers by this authorPhillip J. Bailey Dr.
The Department of Chemistry The King's Buildings, The University of Edinburgh West Mains Road, Edinburgh, Lothian, Scotland, EH9 3JJ (UK) Fax: (+44) 131-650-6452
Search for more papers by this authorSimon Parsons Dr.
The Department of Chemistry The King's Buildings, The University of Edinburgh West Mains Road, Edinburgh, Lothian, Scotland, EH9 3JJ (UK) Fax: (+44) 131-650-6452
Search for more papers by this authorPeter A. Tasker Prof.
The Department of Chemistry The King's Buildings, The University of Edinburgh West Mains Road, Edinburgh, Lothian, Scotland, EH9 3JJ (UK) Fax: (+44) 131-650-6452
Search for more papers by this authorThis work was supported by Avecia, Seiko-Epson Corporation, and EPSRC funding. We would like to thank Stephen Boyer of the Computing and Engineering Department of the University of North London for performing microanalyses. We would also like to thank Dr. Mary McPartlin for a stimulating discussion with regard to interpretation of our crystallographic results.
Graphical Abstract
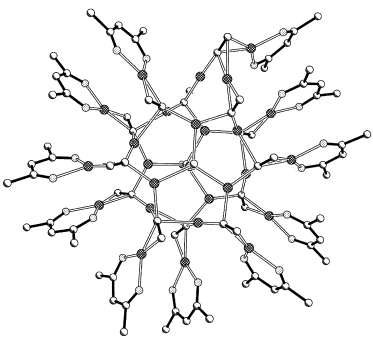
A remarkably anisotropic structure is exhibited by [Cu26(hfac)11(1-pentynyl)15] (hfac=hexafluoroacetylacetone), the largest CuI cluster to have been isolated (see picture). A variety of copper–alkynyl bridging modes are found within the disc-shaped cluster, with all but one of the 26 Cu atoms being located in either of two “layers”. Many short CuI⋅⋅⋅CuI interactions can be discerned within the cluster.
References
- 1D. M. Knotter, A. L. Spek, G. Vankoten, J. Chem. Soc. Chem. Commun. 1989, 1738.
- 2V. W. W. Yam, K. K. W. Lo, K. M. C. Wong, J. Organomet. Chem. 1999, 578, 3.
- 3V. W. W. Yam, K. K. W. Lo, W. K. M. Fung, C. R. Wang, Coord. Chem. Rev. 1998, 171, 17.
- 4V. W. W. Yam, K. K. W. Lo, Chem. Soc. Rev. 1999, 28, 323.
- 5V. W. W. Yam, W. K. Lee, T. F. Lai, Organomet. 1993, 12, 2383.
- 6V. W. W. Yam, W. K. Lee, K. K. Cheung, H. J. Lee, W. P. Leung, J. Chem. Soc. Dalton Trans. 1996, 2889.
- 7V. W. W. Yam, W. K. Lee, K. K. Cheung, B. Crystall, D. Phillips, J. Chem. Soc. Dalton Trans. 1996, 3283.
- 8V. W. W. Yam, W. K. Lee, K. K. Cheung, J. Chem. Soc. Dalton Trans. 1996, 2335.
- 9V. W. W. Yam, C. H. Lam, K. K. Cheung, Inorg. Chim. Acta 2001, 316, 19.
- 10V. W. W. Yam, W. K. M. Fung, M. T. Wong, Organometallics 1997, 16, 1772.
- 11V. W. W. Yam, W. K. M. Fung, K. K. Cheung, J. Cluster Sci. 1999, 10, 37.
- 12V. W. W. Yam, W. K. M. Fung, K. K. Cheung, Organometallics 1998, 17, 3293.
- 13V. W. W. Yam, W. K. M. Fung, K. K. Cheung, Chem. Commun. 1997, 963.
- 14V. W. W. Yam, W. K. M. Fung, K. K. Cheung, Angew. Chem. 1996, 108, 1213; Angew. Chem. Int. Ed. Engl. 1996, 35, 1100.
- 15V. W. W. Yam, S. W. K. Choi, C. L. Chan, K. K. Cheung, Chem. Commun. 1996, 2067.
- 16V. W. W. Yam, J. Photochem. Photobiol. A 1997, 106, 75.
- 17C. R. Wang, K. K. W. Lo, W. K. M. Fung, V. W. W. Yam, Chem. Phys. Lett. 1998, 296, 505.
- 18H. B. Song, Q. M. Wang, Z. Z. Zhang, T. C. W. Mak, Chem. Commun. 2001, 1658.
- 19J. Diez, M. P. Gamasa, J. Gimeno, A. Aguirre, S. Garcia Granda, Organometallics 1991, 10, 380.
- 20J. Diez, M. P. Gamasa, J. Gimeno, A. Aguirre, S. Garcia Granda, Organometallics 1997, 16, 3684.
- 21J. Diez, M. P. Gamasa, J. Gimeno, E. Lastra, A. Aguirre, S. Garcia Granda, Organometallics 1993, 12, 2213.
- 22K. Osakada, T. Takizawa, T. Yamamoto, Organometallics 1995, 14, 3531.
- 23F. Olbrich, J. Kopf, E. Weiss, Angew. Chem. 1993, 105, 1136; Angew. Chem. Int. Ed. Engl. 1993, 32, 1077.
- 24M. P. Gamasa, J. Gimeno, E. Lastra, X. Solans, J. Organomet. Chem. 1988, 346, 277.
- 25D. M. Knotter, A. L. Spek, D. M. Grove, G. Vankoten, Organometallics 1992, 11, 4083.
- 26L. Naldini, F. Demartin, M. Manassero, M. Sansoni, G. Rassu, M. A. Zoroddu, J. Organomet. Chem. 1985, 279, C 42.
- 27M. G. B. Drew, F. S. Esho, S. M. Nelson, J. Chem. Soc. Chem. Commun. 1982, 1347.
- 28
- 28aCrystal structure analysis for [Cu26(hfac)11(1-pentynyl)15]: Crystals were grown by slow cooling of a supersaturated solution of the complex in hot n-hexane and were analyzed with a Bruker smart APEX CCD area detector equipped with an Oxford Cryosystems LT device operating at 150 K. C133H115Cu26F66O22, λ=0.71073 Å, crystal size=0.44×0.38×0.29 mm, cell determination: range 4°<2θ<58°, no. reflections: 4764, monoclinic, space group Cc (checked with MISSYM[28b-d]), Z=4, a=26.009(3), b=21.114(2), c=31.078(4) Å, β=94.214(2)o, V=17 021(3) Å3, ρcalcd=1.943 g cm−3, μ=3.300 mm−1. 75 941 reflections were collected in the range 1.31°<θ<28.94 °, of which 38 578 were unique and 33 027 had Fo>4σ(Fo). A multiscan absorption correction was applied using Sadabs[28e] (Tmax=0.862; Tmin=0.679). The structure was solved by direct methods (SHELXS-97),[28f] and refined by block-matrix least-squares against F2 (SHELXTL97).[28f] All non-hydrogen atoms were modeled with anisotropic displacement parameters, except for one half-occupancy hexane solvate molecule, and H atoms were included in ideal positions. Numerous CF3 groups were disordered, a combination of similarity and ADP equalization restraints were used to model this. The conventional R1 factor (Fo>4σ(Fo)) was 0.0409 and wR2 was 0.0913. (w=1/[σ2(F
 ) + (0.0490 P)2] where P=(F
) + (0.0490 P)2] where P=(F + 2
+ 2  )/3). Largest max/min residual electron density: +1.094/−0.917 e Å3;
)/3). Largest max/min residual electron density: +1.094/−0.917 e Å3;
- 28bY. LePage, J. Appl. Crystallogr. 1999, 20, 264;
- 28cA. L. Spek, J. Appl. Cryst. 1987, 21, 578;
- 28dL. J. Farrugia, J. Appl. Crystallogr. 1999, 32, 837;
- 28eG. M. Sheldrick, SADABS, Program for Empirical Correction of Area Detector Data, University of Göttingen, Göttingen (Germany), 1998;
- 28fG. M. Sheldrick, SHELXTL97, Program for the refinement of crystal structures, University of Göttingen, Göttingen (Germany), 1997. CCDC-186669 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 29V. W. W. Yam, K. L. Yu, K. K. Cheung, J. Chem. Soc. Dalton Trans. 1999, 2913.
- 30V. W. W. Yam, S. H. F. Chong, K. M. C. Wong, K. K. Cheung, Chem. Commun. 1999, 1013.
- 31C.-M. Che, Z. Mao, V. M. Miskowski, M.-C. Tse, C.-K. Chan, K.-K. Cheung, D. L. Phillips, K.-H. Leung, Angew. Chem. 2000, 112, 4250;
Angew. Chem. Int. Ed. 2000, 39, 4084.
10.1002/1521-3773(20001117)39:22<4084::AID-ANIE4084>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 32J. M. Zuo, M. Kim, M. O'Keeffe, J. C. H. Spence, Nature 1999, 401, 49.
- 33H. L. Hermann, G. Boche, P. Schwerdtfeger, Chem. Eur. J. 2001, 7, 5333.
10.1002/1521-3765(20011217)7:24<5333::AID-CHEM5333>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 34L. Magnko, M. Schweizer, G. Rauhut, M. Schutz, H. Stoll, H. J. Werner, Phys. Chem. Chem. Phys. 2002, 4, 1006.
- 35J. C. Slater, J. Chem. Phys. 1964, 41, 3199.
- 36Pairs of atoms in the second annulus defining the parallel sides of the pseudotrigonal prism make contact with the same pair of atoms in the third annulus, namely Cu(2) and Cu(3) with Cu(9) and Cu(10); Cu(4) and Cu(5) with Cu(11) and Cu(12); and Cu(6) and Cu(7) with Cu(8) and Cu(13).
- 37The closest Cu⋅⋅⋅Cu contacts formed by atoms in the “bud unit” (Figure 1) are either longer than the sum of van der Waals radii of two CuI atoms or, for Cu(24)⋅⋅⋅Cu(11) and Cu(24)⋅⋅⋅Cu(25), close to this value (2.7401(9) Å and 2.7057(10) Å respectively).
- 38K. M. Chi, H. K. Shin, M. J. Hampden-Smith, T. T. Kodas, E. N. Duesler, Inorg. Chem. 1991, 30, 4293.
- 39P. Doppelt, T. H. Baum, J. Organomet. Chem. 1996, 517, 53.
- 40R. B. Grossman, Tetrahedron 1999, 55, 919.
- 41I. A. Koppel, J. Koppel, V. Pihl, I. Leito, M. Mishima, V. M. Vlasov, L. M. Yagupolskii, R. W. Taft, J. Chem. Soc. Perkin Trans. 2 2000, 6, 1125.
- 42J. Vicente, M.-T. Chicote, Inorg. Synth. 1998, 32, 172.



41:16<>1.0.co;2-7.cover.gif)
