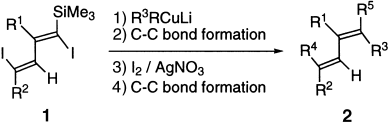
Efficient and General Synthetic Approach to Pentasubstituted Conjugated Dienes Using Site-Selective Coupling of Cuprates with 1,4-Diiodo-1,3-alkadienes as the Key Reaction†
C.D. would like to thank the Japan Society for the Promotion of Science (JSPS) for financial support.
Graphical Abstract
Taking advantage of the presence of a silyl group at the α position in 1, carbon–carbon bonds are formed regioselectively by the reaction of 1 with organocuprate compounds. This method is a practical and efficient approach to molecules of type 2 (R1–R5=alkyl, aryl) with a variety of substitution patterns.



41:16<>1.0.co;2-7.cover.gif)
