Atom-Transfer Tandem Radical Cyclization Reactions Promoted by Lewis Acids†
Dan Yang Prof.
Department of Chemistry, The University of Hong Kong Pokfulam Road, Hong Kong (P. R. China) Fax: (+852) 2859-2159
Search for more papers by this authorShen Gu
Department of Chemistry, The University of Hong Kong Pokfulam Road, Hong Kong (P. R. China) Fax: (+852) 2859-2159
Search for more papers by this authorYi-Long Yan
Department of Chemistry, The University of Hong Kong Pokfulam Road, Hong Kong (P. R. China) Fax: (+852) 2859-2159
Search for more papers by this authorHong-Wu Zhao
Department of Chemistry, The University of Hong Kong Pokfulam Road, Hong Kong (P. R. China) Fax: (+852) 2859-2159
Search for more papers by this authorNian-Yong Zhu
Department of Chemistry, The University of Hong Kong Pokfulam Road, Hong Kong (P. R. China) Fax: (+852) 2859-2159
Search for more papers by this authorDan Yang Prof.
Department of Chemistry, The University of Hong Kong Pokfulam Road, Hong Kong (P. R. China) Fax: (+852) 2859-2159
Search for more papers by this authorShen Gu
Department of Chemistry, The University of Hong Kong Pokfulam Road, Hong Kong (P. R. China) Fax: (+852) 2859-2159
Search for more papers by this authorYi-Long Yan
Department of Chemistry, The University of Hong Kong Pokfulam Road, Hong Kong (P. R. China) Fax: (+852) 2859-2159
Search for more papers by this authorHong-Wu Zhao
Department of Chemistry, The University of Hong Kong Pokfulam Road, Hong Kong (P. R. China) Fax: (+852) 2859-2159
Search for more papers by this authorNian-Yong Zhu
Department of Chemistry, The University of Hong Kong Pokfulam Road, Hong Kong (P. R. China) Fax: (+852) 2859-2159
Search for more papers by this authorThis work was supported by The University of Hong Kong and the Hong Kong Research Grants Council. D.Y. acknowledges the Bristol-Myers Squibb Foundation for an Unrestricted Grant in Synthetic Organic Chemistry and the Croucher Foundation for a Croucher Senior Research Fellowship.
Graphical Abstract
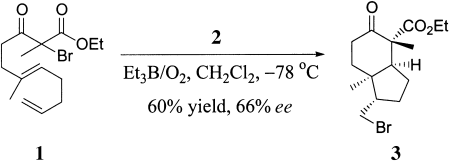
Various polycyclic ring skeletons (e.g. 3) are formed from unsaturated α-bromo β-keto esters (e.g. 1) in a Lewis acid catalyzed atom-transfer tandem radical-cyclization reaction in moderate to good yields and with excellent stereoselectivities. Furthermore, in the presence of chiral complexes such as [Yb(Ph-pybox)(OTf)3] (2), the enantioselective cyclization gave up to 84 % ee. OTf = trifluoromethanesulfonate, pybox = 2,6-bis(2-oxazolin-2-yl)pyridine.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2002/z18697_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews on tandem radical cyclization reactions, see:
- 1aM. Malacria, Chem. Rev. 1996, 96, 289;
- 1bA. J. McCarroll, J. C. Walton, Angew. Chem. 2001, 113, 2282;
10.1002/1521-3757(20010618)113:12<2282::AID-ANGE2282>3.0.CO;2-F Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2224; for recent examples, see:10.1002/1521-3773(20010618)40:12<2224::AID-ANIE2224>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 1cD. P. Curran, J. Sisko, A. Balog, N. Sonoda, K. Nagahara, I. Ryu, J. Chem. Soc. Perkin Trans. 1 1998, 10, 1591;
- 1dS. Kim, J. H. Cheong, J. Yoo, Synlett 1998, 981;
- 1eD. Yang, X.-Y. Ye, S. Gu, M. Xu, J. Am. Chem. Soc. 1999, 121, 5579;
- 1fM. Kizil, B. Patro, O. Callaghan, J. A. Murphy, M. B. Hursthouse, D. Hibbs, J. Org. Chem. 1999, 64, 7856;
- 1gC.-K. Sha, F.-K. Lee, C.-J. Chang, J. Am. Chem. Soc. 1999, 121, 9875;
- 1hB. Patro, J. A. Murphy, Org. Lett. 2000, 2, 3599;
- 1iS. T. Hiton, K. Jones, C. T. Tim, G. Pljevaljcic, M. Schulte, Chem. Commun. 2001, 209;
- 1jC. Ollivier, P. Renaud, J. Am. Chem. Soc. 2001, 123, 4717;
- 1kD. Yang, M. Xu, Org. Lett. 2001, 3, 1785;
- 1lJ. T. Njardarson, I. M. McDonald, D. A. Spiegel, M. Inoue, J. L. Wood, Org. Lett. 2001, 3, 2435;
- 1mY. Haruo, T. Hasegawa, H. Tanaka, T. Takahashi, Synlett 2001, 1935;
- 1nS. Zhou, S. Bommezijn, J. A. Murphy, Org. Lett. 2002, 4, 443.
- 2For recent reviews on free radical reactions in the synthesis of natural products, see:
- 2aC. P. Jasperse, D. P. Curran, T. L. Fevig, Chem. Rev. 1991, 91, 1237;
- 2bP. J. Parsons, C. S. Penkett, A. J. Shell, Chem. Rev. 1996, 96, 195.
- 3For a recent review on enantioselective radical reactions, see:
- 3aM. P. Sibi, N. A. Porter, Acc. Chem. Res. 1999, 32, 163; for recent examples, see:
- 3bU. Iserloh, D. P. Curran, S. Kanemasa, Tetrahedron: Asymmetry 1999, 10, 2417;
- 3cM. P. Sibi, J. Chen, J. Am. Chem. Soc. 2001, 123, 9472;
- 3dM. P. Sibi, Y. Asano, J. B. Sausker, Angew. Chem. 2001, 113, 1333;
10.1002/1521-3757(20010401)113:7<1333::AID-ANGE1333>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1293;10.1002/1521-3773(20010401)40:7<1293::AID-ANIE1293>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 3eM. P. Sibi, J. B. Sausker, J. Am. Chem. Soc. 2002, 124, 984.
- 4D. Yang, S. Gu, Y.-L. Yan, N.-Y. Zhu, K.-K. Cheung, J. Am. Chem. Soc. 2001, 123, 8612.
- 5See: D. P. Curran, T. M. Morgan, C. E. Schwartz, B. B. Snider, M. A. Dombroski, J. Am. Chem. Soc. 1991, 113, 6607; the atom-transfer cyclization of substrates 1 c and 1 d by using (Me3Sn)2/hν as the radical initiator in the absence of Lewis acid has been reported; cyclization of 1 c afforded the same products; both trans and cis products (9:1) were obtained in the cyclization of 1 d.
- 6
- 6aFor an excellent review of Lewis acid mediated radical reactions, see: P. Renaud, M. Gerster, Angew. Chem. 1998, 110, 2704;
10.1002/(SICI)1521-3757(19981002)110:19<2704::AID-ANGE2704>3.0.CO;2-8 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2562; for recent examples of Lewis acid promoted atom-transfer radical reactions, see:10.1002/(SICI)1521-3773(19981016)37:19<2562::AID-ANIE2562>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 6bC. L. Mero, N. A. Porter, J. Am. Chem. Soc. 1999, 121, 5155;
- 6cA. J. Clark, F. D. Campo, R. J. Deeth, R. P. Filik, S. Gatard, N. A. Hunt, D. Lastecoueres, G. H. Thomas, J.-B. Verlhac, H. Wongtap, J. Chem. Soc. Perkin Trans. 1 2000, 671;
- 6dO. Kitagawa, H. Fujiwara, T. Taguchi, Tetrahedron Lett. 2001, 42, 2165;
- 6eO. Kitagawa, Y. Yamada, H. Fujiwara, T. Taguchi, J. Org. Chem. 2002, 67, 922.
- 7For details about side products, see Supporting Information.
- 8For examples on the use of 4-Å molecular sieves in asymmetric reactions, see:
- 8aR. M. Hanson, K. B. Sharpless, J. Org. Chem. 1986, 51, 1922;
- 8bK. Narasaka, N. Iwasawa, M. Inoue, T. Yamada, M. Nakashima, J. Sugimoto, J. Am. Chem. Soc. 1989, 111, 5340;
- 8cK. Mikami, M. Terada, T. Nakai, J. Am. Chem. Soc. 1990, 112, 3949;
- 8dD. A. Evans, M. M. Faul, M. T. Bilodeau, B. A. Anderson, D. M. Barnes, J. Am. Chem. Soc. 1993, 115, 5328;
- 8eT. Iida, N. Yamamoto, H. Sasai, M. Shibasaki, J. Am. Chem. Soc. 1997, 119, 4783.
- 9For recent reviews on lanthanide Lewis acids catalysis, see:
- 9aS. Kobayashi, Synlett 1994, 689;
- 9bM. Shibasaki, K.-I. Yamada, N. Yoshikawa in Lewis Acids in Organic Synthesis, Vol. 2 (Ed: ), Wiley, New York, 2000, chap. 20, p. 911.
- 10For recent reviews on the use of C2-symmetric chiral bisoxazoline ligands in asymmetric catalysis, see:
- 10aA. Pfaltz, Acta Chem. Scand. 1996, 50, 189;
- 10bA. K. Ghosh, P. Mathivanan, J. Cappiello, Tetrahedron: Asymmetry 1998, 9, 1;
- 10cJ. S. Johnson, D. A. Evans, Acc. Chem. Res. 2000, 33, 325.
- 11
- 11aFor a recent review on the effect of additives on asymmetric reactions, see: E. M. Vogl, H. Gröger, M. Shibasaki, Angew. Chem. 1999, 111, 1672;
10.1002/(SICI)1521-3757(19990601)111:11<1672::AID-ANGE1672>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1570; for examples of the additive effect in chiral ytterbium triflate mediated asymmetric reactions, see:10.1002/(SICI)1521-3773(19990601)38:11<1570::AID-ANIE1570>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 11bS. Kobayashi, H. Ishitani, J. Am. Chem. Soc. 1994, 116, 4083;
- 11cS. Kobayashi, H. Ishitani, I. Hachiya, M. Araki, Tetrahedron 1994, 50, 11 623;
- 11dT. Saito, M. Kawamura, J.-I. Nishimura, Tetrahedron Lett. 1997, 38, 3231;
- 11eM. Kawamura, S. Kobayashi, Tetrahedron Lett. 1999, 40, 3213.
- 12D. A. Evans, Z. K. Sweeney, T. Rovis, J. S. Tedrow, J. Am. Chem. Soc. 2001, 123, 12 095.



41:16<>1.0.co;2-7.cover.gif)
