Two [Nb6Cl9O3(CN)6]5− Isomer Anions in Two Nb6 Cluster Oxyhalides: Cs5[Nb6Cl9O3(CN)6]⋅4 H2O and (Me4N)5[Nb6Cl9O3(CN)6]⋅5 H2O†
Nikolai G. Naumov Dr.
Institute of Inorganic Chemistry, SB RAS Ak. Lavrentiev prosp. 3 Novosibirsk 630090 (Russia) Fax: (+7) 3832-344-489
Search for more papers by this authorStephane Cordier Dr.
Institut de Chimie de Rennes Laboratoire de Chimie du Solide et Inorganique Moléculaire UMR 6511 CNRS-Université de Rennes 1 Avenue du Général Leclerc 35042 Rennes cedex (France)
Search for more papers by this authorChristiane Perrin Dr.
Institut de Chimie de Rennes Laboratoire de Chimie du Solide et Inorganique Moléculaire UMR 6511 CNRS-Université de Rennes 1 Avenue du Général Leclerc 35042 Rennes cedex (France)
Search for more papers by this authorNikolai G. Naumov Dr.
Institute of Inorganic Chemistry, SB RAS Ak. Lavrentiev prosp. 3 Novosibirsk 630090 (Russia) Fax: (+7) 3832-344-489
Search for more papers by this authorStephane Cordier Dr.
Institut de Chimie de Rennes Laboratoire de Chimie du Solide et Inorganique Moléculaire UMR 6511 CNRS-Université de Rennes 1 Avenue du Général Leclerc 35042 Rennes cedex (France)
Search for more papers by this authorChristiane Perrin Dr.
Institut de Chimie de Rennes Laboratoire de Chimie du Solide et Inorganique Moléculaire UMR 6511 CNRS-Université de Rennes 1 Avenue du Général Leclerc 35042 Rennes cedex (France)
Search for more papers by this authorThis work was supported by INTAS (grant N2000-00689). N.G.N. is grateful to the NATO for financial support during his stay at the LCSIM. The authors thank the Center of Diffractometry of Rennes 1 University for crystal structures and the Center for Scanning Electron Microscopy and Microanalyses of Rennes 1 University for analyses.
Graphical Abstract
References
- 1
- 1aJ. R. Long, L. S. McCarty, R. H. Holm, J. Am. Chem. Soc. 1996, 118, 4603–4616;
- 1bN. Prokopuk, D. F. Shriver, Adv. Inorg. Chem. 1999, 46, 1–49;
- 1cT. Saito, J. Chem. Soc. Dalton Trans. 1999, 97–105, and references herein.
- 2A. Simon, Clusters and Colloids. From Theory to Applications (Ed.: ), VCH, Weinheim, 1994.
- 3J. D. Corbett, J. Alloys Compd. 1995, 229, 10–23.
- 4C. Perrin in Metal Cluster in Chemistry, Vol. 3 (Ed ), Wiley-VCH, Weinheim, 1999, pp. 1563–1590.
10.1002/9783527618316.ch5e Google Scholar
- 5C. Perrin, R. Chevrel, M. Sergent, O. Fischer, Mater. Res. Bull. 1979, 14, 1505–1515.
- 6C. Perrin, M. Potel, M. Sergent, Acta Crystallogr. Sect. C. 1983, 39, 415–418.
- 7C. Perrin, M. Sergent, F. Le Traon, A. Le Traon, J. Solid State Chem. 1978, 25, 197–204.
- 8A. Perricone, A. Slougui, A. Perrin, Solid State Sci. 1999, 1, 657–666.
- 9J. C. P. Gabriel, K. Boubekeur, S. Uriel, P. Batail, Chem. Rev 2001, 101, 2037–2066, and references herein.
- 10S. Cordier, F. Gulo, C. Perrin, Solid State Sci. 1999, 1, 637–646.
- 11
- 11aS. Cordier, C. Perrin, M. Sergent, Eur. J. Solid State Inorg. Chem. 1994, 31, 1049–1060;
- 11bS. Cordier, C. Perrin, M. Sergent, J. Solid State Chem. 1995, 120, 43–48;
- 11cS. Cordier, C. Perrin, M. Sergent, Mater. Res. Bull. 1996, 31, 683–690;
- 11dS. Cordier, C. Perrin, M. Sergent, Mater. Res. Bull. 1997, 32, 25–33;
- 11eF. Gulo, C. Perrin, J. Mater. Chem. 2000, 10, 1721–1724;
- 11fF. Gulo, T. Roisnel, C. Perrin, J. Mater. Chem. 2001, 11, 1237–1241;
- 11gF. Gulo, C. Perrin, J. Solid State Chem., in press.
- 12
- 12aE. V. Anokhina, M. W. Essig, A. Lachgar, Angew. Chem. 1998, 110, 538–540;
10.1002/(SICI)1521-3757(19980216)110:4<538::AID-ANGE538>3.0.CO;2-3 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 522–525;10.1002/(SICI)1521-3773(19980302)37:4<522::AID-ANIE522>3.0.CO;2-Y CAS Web of Science® Google Scholar
- 12bE. V. Anokhina, C. S. Day, A. Lachgar, Chem. Commun. 2000, 1491–1492;
- 12cE. V. Anokhina, C. S. Day, M. W. Essig, A. Lachgar, Angew. Chem. 2000, 112, 1089–1091;
10.1002/(SICI)1521-3757(20000317)112:6<1089::AID-ANGE1089>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1047–1049;10.1002/(SICI)1521-3773(20000317)39:6<1047::AID-ANIE1047>3.0.CO;2-E CAS PubMed Web of Science® Google ScholarE. V. Anokhina, C. S. Day, A. Lachgar, Inorg. Chem. 2001, 40, 5072–5076.
- 13N. R. M. Crawford, J. R. Long, Inorg. Chem. 2001, 40, 3456–3462.
- 14
- 14aN. G. Naumov, A. V. Virovets, M. N. Sokolov, S. B. Artemkina, V. E. Fedorov, Angew. Chem. 1998, 110, 2043–2045;
10.1002/(SICI)1521-3757(19980703)110:13/14<2043::AID-ANGE2043>3.0.CO;2-G Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1943–1945;10.1002/(SICI)1521-3773(19980803)37:13/14<1943::AID-ANIE1943>3.0.CO;2-Q CAS Web of Science® Google Scholar
- 14bN. G. Naumov, D. V. Soldatov, J. A. Ripmeester, S. B. Artemkina, V. E. Fedorov, Chem. Commun. 2001, 571–572, and references herein;
- 14cM. V. Bennett, L. G. Beauvais, M. P. Shores, J. R. Long, J. Am. Chem. Soc. 2001, 123, 8022–8032, and references herein.
- 15For M=Re:
- 15aY. V. Mironov, J. A. Cody, T. E. Albrecht-Schmitt, J. A. Ibers, J. Am. Chem. Soc. 1997, 119, 493–498;
- 15bN. G. Naumov, A. V. Virovets, N. V. Podberezskaya, V. E. Fedorov, J. Struct. Chem. 1997, 38, 857–862;
- 15cH. Imoto, N. G. Naumov, A. V. Virovets, T. Saito, V. E. Fedorov, J. Struct. Chem. 1998, 39, 720–727;
- 15dV. E. Fedorov, S. V. Tkachev, N. G. Naumov, Yu. V. Mironov, Yu. I. Mironov, Russ. J. Inorg. Chem. 1998, 43, 1562–1572;
- 15eL. G. Beauvais, M. P. Shores, J. R. Long, Chem. Mater. 1998, 10, 3783–3786; for M=Mo:
- 15fY. V. Mironov, A. V. Virovets, N. G. Naumov, V. N. Ikorskii, V. E. Fedorov, Chem. Eur. J. 2000, 6, 1361–1365;
10.1002/(SICI)1521-3765(20000417)6:8<1361::AID-CHEM1361>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 15gK. A. Brylev, A. V. Virovets, N. G. Naumov, Yu. V. Mironov, D. Fenske, V. E. Fedorov, Russ. Chem. Bull. 2001, 50, 1140–1143;
- 15hC. Magliocchi, X. B. Xie, T. Hughbanks, Inorg. Chem. 2000, 39, 5000–5001; for M=W:
- 15iS. Jin, F. J. DiSalvo, Chem. Commun. 2001, 1586–1587.
- 16J. Koehler, G. Svensson, A. Simon, Angew. Chem. 1992, 104, 1463–1483; Angew. Chem. Int. Ed. Engl. 1992, 31, 1437–1456.
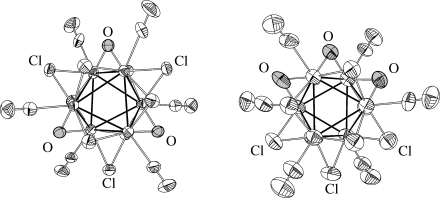
- 17This distortion was reflected by the value for the C-X-C angle (where X is the center of mass for the Nb6 octahedron). These angles differ for NbNb edges bridged by μ-O and μ-Cl atoms and are in the ranges of 79.5–82.6 ° (μ-O) and 92.0–94.0° (μ-Cl) for 1 and 80.5–85.9 ° (μ-O) and 87.5–94.1° (μ-O) for 2.
- 18aM. B. Hursthouse, A. M. Galas, J. Chem. Soc. Chem. Commun. 1980, 1167–1168;
- 18bM. Laing, G. Gafner, W. P. Griffith, P. M. Kiernan, Inorg. Chim. Acta 1979, 33, L 119–120;
- 18cV. P. Fedin, I. V. Kalinina, A. V. Virovets, N. V. Podberezskaya, I. S. Neretin, Yu. L. Slovokhotov, Chem. Commun. 1998, 2579–2580.
- 19F. Ogliaro, S. Cordier, J. F. Halet, C. Perrin, J. Y. Saillard, M. Sergent, Inorg. Chem. 1998, 37, 6199–6207.
- 20
- 20aK. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed., Wiley, New York, 1997, and references herein;
- 20bK. R. Dunbar, R. A. Heintz, Prog. Inorg. Chem. 1997, 45, 283–391, and references herein.
- 21
- 21aG. M. Sheldrick, SHELXS-97: Program for the Solution of Crystal Structures, University of Göttingen, Göttingen (Germany), 1990;
- 21bG. M. Sheldrick, SHELXL-97: Program for the Refinement of Crystal Structures, University of Göttingen, Göttingen (Germany), 1997.
- 22CCDC-175878 and CCDC-175879 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).



41:16<>1.0.co;2-7.cover.gif)
