1,2,3,5-Tetrahydro-1,2,3-methenopentalene, a Valence Isomer of Isoindene: Synthesis and Diels–Alder Reactions†
This work was supported by the Fonds der Chemischen Industrie and by Chemetall GmbH. S.E.L. and D.D.P. thank Heriot-Watt University, Edinburgh, and the European Community for an Erasmus exchange scholarship. Dipl.-Chem. Patrick Musch assisted us in the performance of the quantum-chemical calculations.
Graphical Abstract
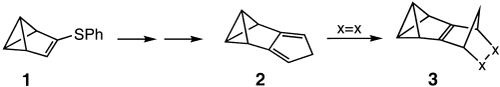
A strong pyramidalization of the double bond in the Diels–Alder adducts 3, as suggested by quantum-chemical calculations and NMR spectroscopic data, is probably the reason why these very reactive compounds are observable at best at low temperatures. The title compound 2, required for preparation of 3, was prepared in five steps from benzvalene 1.



41:16<>1.0.co;2-7.cover.gif)
