Boron Chemistry Lights the Way: Optical Properties of Molecular and Polymeric Systems†
Christopher D. Entwistle
Department of Chemistry University of Durham South Road, Durham DH1 3LE (UK) Fax: (+44) 191-386-1127
Search for more papers by this authorTodd B. Marder Prof. Dr.
Department of Chemistry University of Durham South Road, Durham DH1 3LE (UK) Fax: (+44) 191-386-1127
Search for more papers by this authorChristopher D. Entwistle
Department of Chemistry University of Durham South Road, Durham DH1 3LE (UK) Fax: (+44) 191-386-1127
Search for more papers by this authorTodd B. Marder Prof. Dr.
Department of Chemistry University of Durham South Road, Durham DH1 3LE (UK) Fax: (+44) 191-386-1127
Search for more papers by this authorC.D.E. thanks EPSRC for a postgraduate studentship and Syngenta for a postgraduate scholarship, and T.B.M. thanks the University of Durham for support and Prof. Dr. K. Tamao for a preprint of ref. 32.
Graphical Abstract
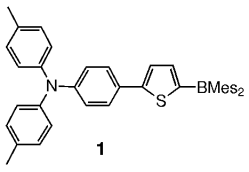
Molecular and polymeric boron-containing systems are proving to display interesting optical and electrooptical properties. Molecules such as thiophene 1 with a donor (4-[bis(4-methylphenyl)amino]phenyl) and an acceptor (dimesitylboryl) substituent, are strongly electroluminescent, and have been incorporated into organic electronic devices.
Abstract
Electrooptical and electronic materials are the subject of much research interest, whereby the focus has often been on electron-rich organic molecules. In the past years, new routes to electron-deficient systems have been developed that take advantage of the fact that three-coordinate boron is isoelectronic and isostructural with a positively charged carbocation, which allows neutral, p-type materials to be synthesized directly. This minireview summarizes recent work on compounds with 3- and 4-coordinate boron as well as boron clusters, placing it in the context of prior studies by the research groups of Williams and Glowgowski, Kaim, Lequan, and Marder.
References
- 1
- 1a “Nonlinear Optical Properties of Organic and Polymeric Materials”: ACS Symp. Ser. 1983, 233;
- 1b Nonlinear Optical Properties of Organic Molecules and Crystals ( ), Academic Press, New York, 1987;
- 1c P. N. Prasad, D. J. Williams, Introduction to Nonlinear Optical Effects in Molecules and Polymers, Wiley, New York, 1991.
- 2
- 2a P. May, Phys. World 1995, 52–57;
- 2b D. D. C. Bradley, Curr. Opin. Solid State Mater. Sci. 1996, 1, 789–797;
- 2c A. B. Holmes, D. D. C. Bradley, A. R. Brown, P. L. Burn, J. H. Burroughes, R. H. Friend, N. C. Greenham, R. W. Gymer, D. A. Halliday, R. W. Jackson, A. Kraft, J. H. F. Martens, K. Pilcher, I. D. W. Samuel, Synth. Met. 1993, 55–57, 4031–4040.
- 3 U. Mitschke, P. Bäuerle, J. Mater. Chem. 2000, 10, 1471–1507.
- 4 M. Rumi, J. E. Ehrlich, A. A. Heikal, J. W. Perry, S. Barlow, Z. Hu, D. McCord-Maughon, T. C. Parker, H. Röckel, S. Thayumanavan, S. R. Marder, D. Beljonne, J.-L. Brédas, J. Am. Chem. Soc. 2000, 122, 9500–9510.
- 5 N. Matsumi, Y. Chujo in Contemporary Boron Chemistry, Spec. Publ. No. 253 ( ), The Royal Society of Chemistry, Cambridge, 2000, pp. 51–58.
- 6
- 6a N. Matsumi, K. Naka, Y. Chujo, J. Am. Chem. Soc. 1998, 120, 5112–5113;
- 6b N. Matsumi, M. Miyata, Y. Chujo, Macromolecules 1999, 32, 4467–4469.
- 7 N. Matsumi, K. Naka, Y. Chujo, J. Am. Chem. Soc. 1998, 120, 10 776–10 777.
- 8
- 8a N. Matsumi, K. Naka, Y. Chujo, Polym. J. 1998, 30, 833–837;
- 8b N. Matsumi, K. Naka, Y. Chujo, Macromolecules 1998, 31, 8047–8050;
- 8c N. Matsumi, T. Umeyama, Y. Chujo, Macromolecules 2001, 43, 3510–3511;
- 8d M. Miyata, N. Matsumi, Y. Chujo, Macromolecules 2001, 34, 7331–7335.
- 9
- 9a N. Matsumi, Y. Chujo, O. Lavaster, P. H. Dixneuf, Organometallics 2001, 20, 2425–2427;
- 9b F. Matsumoto, N. Matsumi, Y. Chujo, Polym. Bull. 2001, 46, 257–262.
- 10 N. Matsumi, Y. Chujo, Polym. J. 2001, 33, 383–386.
- 11
- 11a T. Noda, Y. Shirota, J. Am. Chem. Soc. 1998, 120, 9714–9715;
- 11b T. Noda, H. Ogawa, Y. Shirota, Adv. Mater. 1999, 11, 283–285;
- 11c T. Noda, Y. Shirota, J. Lumin. 2000, 87–89, 1168–1170.
- 12
- 12a Y. Hamada, C. Adachi, T. Tsutsui, S. Saito, Jpn. J. Appl. Phys. 1992, 31, 1812;
- 12b J. Kido, M. Kimura, K. Nagai, Chem. Lett. 1996, 47.
- 13 M. Kinoshita, Y. Shirota, Chem. Lett. 2001, 614–615.
- 14 Y. Shirota, M. Kinoshita, T. Noda, K. Okumuto, T. Ohara, J. Am. Chem. Soc. 2000, 122, 11 021–11 022.
- 15 M. Kinoshita, N. Fujii, T. Tsuzaki, Y. Shirota, Synth. Met. 2001, 121, 1571–1572.
- 16 C. Branger, M. Lequan, R. M. Lequan, M. Barzoukas, A. Fort, J. Mater. Chem. 1996, 6, 555–558.
- 17 R. J. P. Corriu, T. Deforth, W. E. Douglas, G. Guerrero, W. S. Siebert, Chem. Commun. 1998, 963–964.
- 18
- 18a Z. Yuan, J. C. Collings, N. J. Taylor, T. B. Marder, C. Jardin, J.-F. Halet, J. Solid State Chem. 2000, 154, 5–12;
- 18b Z. Yuan, N. J. Taylor, T. B. Marder, I. D. Williams, S. K. Kurtz, L. T. Cheng, Chem. Commun. 1990, 1489–1492;
- 18c Z. Yuan, N. J. Taylor, T. B. Marder, I. D. Williams, L. T. Cheng in Organic Materials for Non-linear Optics II, Spec. Publ. No. 91 ( ), The Royal Society of Chemistry, Cambridge, 1991, pp. 190–194.
- 19 Z. Yuan, N. J. Taylor, R. Ramachandran, T. B. Marder, Appl. Organomet. Chem. 1996, 10, 305–316.
- 20 M. Lequan, R. M. Lequan, K. C. Ching, J. Mater. Chem. 1991, 1, 997–999.
- 21 M. Lequan, R. M. Lequan, K. C. Ching, J. Mater. Chem. 1992, 2, 719–725.
- 22 C. Branger, M. Lequan, R. M. Lequan, M. Large, F. Kajzar, Chem. Phys. Lett. 1997, 272, 265–270.
- 23 R. B. Comizolli, J. Electrochem. Soc. 1987, 134, 424.
- 24 W. Kaim, A. Schulz, Angew. Chem. 1984, 96, 611; Angew. Chem. Int. Ed. Engl. 1984, 23, 615–616.
- 25 A. Schulz, W. Kaim, Chem. Ber. 1989, 122, 1863–1868.
- 26 J. Fiedler, S. Zalis, A. Klein, F. M. Hornung, W. Kaim, Inorg. Chem. 1996, 35, 3039–3043.
- 27 A. Lichtblau, W. Kaim, A. S. Schultz, T. Stahl, J. Chem. Soc. Perkin Trans. 2 1992, 1497–1501.
- 28 J. C. Doty, B. Babb, P. J. Grisdale, M. E. Glogowski, J. L. R. Williams, J. Organomet. Chem. 1972, 38, 229–236.
- 29 M. E. Glogowski, J. L. R. Williams, J. Organomet. Chem. 1981, 216, 1–8.
- 30 K. Albrecht, V. Kaiser, R. Boese, J. Adams, D. E. Kaufmann, J. Chem. Soc. Perkin Trans. 2 2000, 2153–2157.
- 31 S. Yamaguchi, S. Akiyama, K. Tamao, J. Am. Chem. Soc. 2000, 122, 6335–6336.
- 32 S. Yamaguchi, S. Akiyama, K. Tamao, J. Am. Chem. Soc. 2001, 123, 11 372–11 375.
- 33 S. Yamaguchi, T. Shirasaka, K. Tamao, Org. Lett. 2000, 2, 4129–4132.
- 34
- 34a B. Y. Lee, S. Wang, M. Putzer, G. P. Bartholomew, X. Bu, G. C. Bazan, J. Am. Chem. Soc. 2000, 122, 3969–3970;
- 34b B. Y. Lee, G. C. Bazan, J. Am. Chem. Soc. 2000, 122, 8577–8578.
- 35
- 35a
C. Lambert, S. Stadler, G. Bourhill, C. Bräuchle, Angew. Chem. 1996, 108, 710–712;
10.1002/ange.19961080623 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 644–646;
- 35b K. C. Ching, M. Lequan, R. M. Lequan, A. Grisard, D. Markovitsi, J. Chem. Soc. Faraday Trans. 1991, 87, 2225–2228.
- 36 M. J. G. Lesley, A. Woodward, N. J. Taylor, T. B. Marder, I. Cazenobe, I. Ledoux, J. Zyss, A. Thornton, D. W. Bruce, A. Kakkar, Chem. Mater. 1998, 10, 1355–1365.
- 37 Z. M. Su, X. J. Wang, Z. H. Huang, R. S. Wang, J. K. Feng, J. Z. Sun, Synth. Met. 2001, 119, 583–584.
- 38 C. W. Tang, S. A. van Slyke, Appl. Phys. Lett. 1987, 51, 913–915.
- 39
- 39a S. Anderson, M. S. Weaver, A. J. Hudson, Synth. Lett. 2000, 111/112, 459–463;
- 39b Q. Wu, M. Esteghamation, N.-X. Hu, Z. Popovic, G. Enwright, Y. Tao, M. D'Iorio, S. Wang, Chem. Mater. 2000, 12, 79–83.
- 40
- 40a X. T. Tao, H. Suzuki, T. Wada, S. Miyata, H. Sasabe, J. Am. Chem. Soc. 1999, 121, 9447–9448;
- 40b X. T. Tao, H. Suzuki, T. Wada, H. Sasabe, S. Miyata, Appl. Phys. Lett. 1999, 75, 1655–1656.
- 41
- 41a A. Hassan, S. Wang, Chem. Commun. 1998, 211–212;
- 41b
M. Esteghamation, N. X. Hu, Z. Popovic, G. Enright, S. R. Breeze, S. Wang, Angew. Chem. 1999, 38, 1039–1041;
Angew. Chem. Int. Ed. 1999, 38, 985–988.
10.1002/(SICI)1521-3773(19990401)38:7<985::AID-ANIE985>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 42 S. Wang, Coord. Chem. Rev. 2001, 215, 79–98.
- 43 Y. Li, Y. Liu, J. Guo, Y. Wang, Chem. Commun. 2000, 1551–1552.
- 44 See, for example:
- 44a H. Imahori, H. Norieda, H. Yamada, Y. Nishimura, I. Yamazaki, Y. Sakata, S. Fukuzumi, J. Am. Chem. Soc. 2001, 123, 100–110;
- 44b F. Li, S. I. Yang, Y. Ciringh, J. Seth, C. H. Martin, D. L. Singh, D. Kim, R. R. Birge, D. F. Bocian, D. Holten, J. S. Lindsey, J. Am. Chem. Soc. 1998, 120, 10 001–10 017;
- 44c T. Gareis, C. Huber, O. S. Wolfbeis, J. Daub, Chem. Commun. 1997, 1717–1718;
- 44d K. Rurack, M. Kollmannsberger, J. Daub, New J. Chem. 2001, 25, 289–292;
- 44e G. Beer, K. Rurack, J. Daub, Chem. Commun. 2001, 1138–1139.
- 45
- 45a D. M. Murphy, D. M. P. Mingos, J. M. Forward, J. Mater. Chem. 1993, 3, 67–76;
- 45b D. M. Murphy, D. M. P. Mingos, J. L. Haggit, H. R. Rowell, S. A. Westcott, T. B. Marder, N. J. Taylor, D. R. Kanis, J. Mater. Chem. 1993, 3, 139–148.
- 46 B. Grüner, Z. Janoušek, B. T. King, J. N. Woodford, C. H. Wang, V. Všetecka, J. Michl, J. Am. Chem. Soc. 1999, 121, 3122–3126.
- 47
- 47a R. Littger, J. Taylor, G. Rudd, A. Newlon, D. Allis, S. Kotiah, J. T. Spencer in Contemporary Boron Chemistry, Spec. Publ. No. 253 ( ), The Royal Society of Chemistry, Cambridge, 2000, pp. 67–76;
- 47b D. G. Allis, J. T. Spencer, J. Organomet. Chem. 2000, 614, 309–313;
- 47c D. G. Allis, J. T. Spencer, Inorg. Chem, 2001, 40, 3373–3380;
- 47d J. Taylor, J. Caruso, A. Newlon, U. Englisch, K. Ruhlandt-Senge, J. T. Spencer, Inorg. Chem. 2001, 40, 3381–3388.
- 48 J. Abe, N. Nemoto, Y. Nagase, Y. Shirai, T. Iyoda, Inorg. Chem. 1998, 37, 172–173.
- 49 M. Lamrani, R. Hamasaki, M. Mitsuichi, T. Miyashita, Y. Yamamoto, Chem. Commun. 2000, 1595–1596.
- 50 E. Hong, H. Jang, Y. Kim, S. C. Jeoung, Y. Do, Adv. Mater. 2001, 13, 1094–1096.



41:16<>1.0.co;2-7.cover.gif)
