Click Chemistry In Situ: Acetylcholinesterase as a Reaction Vessel for the Selective Assembly of a Femtomolar Inhibitor from an Array of Building Blocks
Warren G. Lewis
Department of Chemistry The Scripps Research Institute 10550 North Torrey Pines Road La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorLuke G. Green Dr.
Department of Chemistry The Scripps Research Institute 10550 North Torrey Pines Road La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorFlavio Grynszpan Prof.
Department of Molecular Biology The Scripps Research Institute 10550 North Torrey Pines Road La Jolla, CA 92037 (USA)
Search for more papers by this authorZoran Radić Dr.
Department of Pharmacology University of California San Diego La Jolla, CA 92093-0636 (USA)
Search for more papers by this authorPaul R. Carlier Prof.
Department of Chemistry Virginia Polytechnic Institute and State University Blacksburg, VA 24061 (USA)
Search for more papers by this authorPalmer Taylor Prof.
Department of Pharmacology University of California San Diego La Jolla, CA 92093-0636 (USA)
Search for more papers by this authorM. G. Finn Prof.
Department of Chemistry The Scripps Research Institute 10550 North Torrey Pines Road La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorK. Barry Sharpless Prof.
Department of Chemistry The Scripps Research Institute 10550 North Torrey Pines Road La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorWarren G. Lewis
Department of Chemistry The Scripps Research Institute 10550 North Torrey Pines Road La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorLuke G. Green Dr.
Department of Chemistry The Scripps Research Institute 10550 North Torrey Pines Road La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorFlavio Grynszpan Prof.
Department of Molecular Biology The Scripps Research Institute 10550 North Torrey Pines Road La Jolla, CA 92037 (USA)
Search for more papers by this authorZoran Radić Dr.
Department of Pharmacology University of California San Diego La Jolla, CA 92093-0636 (USA)
Search for more papers by this authorPaul R. Carlier Prof.
Department of Chemistry Virginia Polytechnic Institute and State University Blacksburg, VA 24061 (USA)
Search for more papers by this authorPalmer Taylor Prof.
Department of Pharmacology University of California San Diego La Jolla, CA 92093-0636 (USA)
Search for more papers by this authorM. G. Finn Prof.
Department of Chemistry The Scripps Research Institute 10550 North Torrey Pines Road La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorK. Barry Sharpless Prof.
Department of Chemistry The Scripps Research Institute 10550 North Torrey Pines Road La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorWe thank the National Institute of General Medical Sciences, National Institutes of Health (GM-28384, K.B.S.; R-37 GM 18360, P.T.), the National Science Foundation (CHE-9985553, K.B.S.), The Skaggs Institute for Chemical Biology (K.B.S., M.G.F.; W.G.L. is a Skaggs Predoctoral Fellow), the W. M. Keck Foundation (K.B.S.), and the J. S. Guggenheim Memorial Foundation (F.G.) for financial support. We are grateful to Dr. Pascale Marchot (University of Marseille, France) for providing us with a purified preparation of Electrophorus electricus AChE for kinetic measurements. We also thank Prof. D. W. Armstrong, C. Mitchell, Dr. G. M. Morris, Dr. X. Wu, Dr. Z. Shen, and Prof. G. Siuzdak for assistance in the execution of this project, and Professors V. V. Fokin and R. Ghadiri for valuable discussions. W.G.L. and L.G.G. contributed equally to this work.
Graphical Abstract
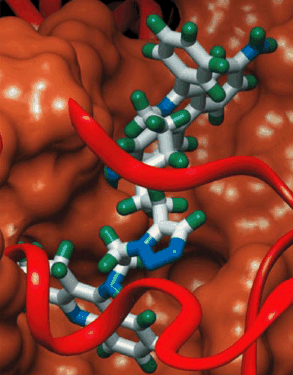
Maßgeschneiderte Chemie in einer Protein-Vorlage: Bei einer Reihe von Aziden und Alkinen, Reaktanten für eine 1,3-dipolare Cycloaddition, wird vom Enzym Acetylcholinesterase bevorzugt ein Paar von Reaktionspartnern umgesetzt, von denen beide an benachbarten Stellen in der Proteinstruktur binden (siehe Bild). Das dabei gebildete 1,2,3-Triazol ist der stärkste bisher entwickelte nichtkovalent gebundene Inhibitor für dieses Enzym.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.com or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 See the following special issues of Curr. Opin. Chem. Biol. devoted to combinatorial chemistry Curr. Opin. Chem. Biol. 2001, 5(3), 229–336 (Eds.: T. Caulfield, K. Burgess); Curr. Opin. Chem. Biol. 2000, 4(3), 243–355 (Eds.: M. Bradley, L. Weber); Curr. Opin. Chem. Biol. 1999, 3(3), 241–356 (Eds.: P. A. Bartlett, G. F. Joyce).
- 2 B. A. Bunin, J. M. Dener, D. A. Livingston, Annu. Rep. Med. Chem. 1999, 34, 267–286.
- 3 S. R. Wilson, A. W. Czarnik, Combinatorial Chemistry, Wiley, New York, 1997.
- 4
- 4a A. V. Eliseev, Curr. Opin. Drug Discovery Dev. 1998, 1, 106–115;
- 4b O. Ramströn, J.-M. Lehn, Nat. Rev. Drug Disc 2002, 1, 26–36.
- 5 J.-M. Lehn, A. V. Eliseev, Science 2001, 291, 2331–2332.
- 6
T. Bunyapaiboonsri, O. Ramström, S. Lohmann, J.-M. Lehn, L. Peng, M. Goeldner, ChemBioChem 2001, 2, 438–444.
10.1002/1439-7633(20010601)2:6<438::AID-CBIC438>3.0.CO;2-J CAS PubMed Web of Science® Google Scholar
- 7 D. J. Maly, I. C. Choong, J. A. Ellman, Proc. Natl. Acad. Sci. USA 2000, 97, 2419–2424.
- 8
K. C. Nicolaou, R. Hughes, S. Y. Cho, N. Winssinger, C. Smethurst, H. Labischinski, R. Endermann, Angew. Chem. 2000, 112, 3981–3986;
10.1002/1521-3757(20001103)112:21<3981::AID-ANGE3981>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3823–3828.10.1002/1521-3773(20001103)39:21<3823::AID-ANIE3823>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 9 S. B. Shuker, P. J. Hajduk, R. P. Meadows, S. W. Fesik, Science 1996, 274, 1531–1534.
- 10 X. Cheng, R. Chen, J. E. Bruce, B. L. Schwartz, G. A. Anderson, S. A. Hofstadler, D. C. Gale, R. D. Smith, J. Gao, G. B. Sigal, M. Mammen, G. M. Whitesides, J. Am. Chem. Soc. 1995, 117, 8859–8860.
- 11 D. C. Rideout, T. Calogeropoulou, in Synergism and Antagonism in Chemotherapy, Academic Press, Orlando, 1991, chap. 14, pp. 507–535.
- 12 A. J. Kirby, Adv. Phys. Org. Chem. 1980, 17, 183–278.
- 13 W. P. Jencks, Proc. Natl. Acad. Sci. USA 1981, 78, 4046–4050.
- 14
M. Mammen, S.-K. Choi, G. M. Whitesides, Angew. Chem. 1998, 110, 2908–2953;
10.1002/(SICI)1521-3757(19981016)110:20<2908::AID-ANGE2908>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2755–2794.10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3 CAS Web of Science® Google Scholar
- 15 J. F. A. Chase, P. K. Tubbs, Biochem. J. 1969, 111, 225–235.
- 16
R. Nguyen, I. Huc, Angew. Chem. 2001, 113, 1824–1826;
Angew. Chem. Int. Ed. 2001, 40, 1774–1776.
10.1002/1521-3773(20010504)40:9<1774::AID-ANIE17740>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 17 J. Inglese, S. J. Benkovic, Tetrahedron 1991, 47, 2351–2364.
- 18 S. E. Greasley, T. H. Marsilje, H. Cai, S. Baker, S. J. Benkovic, D. L. Boger, I. A. Wilson, Biochemistry 2001, 40, 13 538–13 547.
- 19
K. C. Nicolaou, R. Hughes, S. Y. Cho, N. Winssinger, H. Labischinski, R. Endermann, Chem. Eur. J. 2001, 7, 3824–3843.
10.1002/1521-3765(20010903)7:17<3824::AID-CHEM3824>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 20
H. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. 2001, 113, 2056–2075;
Angew. Chem. Int. Ed. 2001, 40, 2004–2021.
10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 21
A. Michael, J. Prakt. Chem. 1893, 48, 94.
10.1002/prac.18930480114 Google Scholar
- 22 R. Huisgen in Profiles, Pathways, and Dreams ( ), American Chemical Society, Washington, DC, 1994.
- 23 In the description of “click chemistry,” (ref. [20.]) we inadvertently omitted mention of the powerful block cycloaddition-connection strategies pioneered by Warrener and co-workers (see, for example, R. N. Warrener, D. N. Butler, Aldrichimica Acta 1997, 30, 119–129; R. N. Warrener, D. N. Butler, D. Margetic, F. M. Pfeffer, R. A. Russel, Tetrahedron Lett. 2000, 41, 4671–4675). This body of work offers many beautiful examples of modular synthetic sequences for the construction of polycyclic skeletons and other interesting structures. Its success relies, both in design and execution, on a handful of concerted cycloaddition reactions—pure “fusion” events that are premier examples of click chemistry.
- 24 E. Saxon, C. R. Bertozzi, Science 2000, 287, 2007–2010.
- 25 K. L. Kiick, E. Saxon, D. A. Tirrell, C. R. Bertozzi, Proc. Natl. Acad. Sci. USA 202, 99, 19–24.
- 26 W. L. Mock, T. A. Irra, J. P. Wepsiec, T. L. Manimaran, J. Org. Chem. 1983, 48, 3619–3620.
- 27 W. L. Mock, T. A. Irra, J. P. Wepsiec, M. Adhya, J. Org. Chem. 1989, 54, 5302–5308.
- 28 W. L. Mock, Top. Curr. Chem. 1995, 175, 1–24.
- 29 See also: J. Chen, J. Rebek, Jr., Org. Lett. 2002, 4, 327–329; C. A. Booth, D. Philp, Tetrahedron Lett. 1998, 39, 6987–6990; S. J. Howell, N. Spencer, D. Philp, Tetrahedron 2001, 57, 4945–4954.
- 30 D. M. Quinn, Chem. Rev. 1987, 87, 955–979.
- 31 P. Taylor, Z. Radić, Annu. Rev. Pharmacol. Toxicol. 1994, 34, 281–320.
- 32 J. L. Sussman, I. Silman, P. H. Axelsen, C. Hirth, M. Goeldner, F. Bouet, L. Ehret-Sabatier, I. Schalk, M. Harel, Proc. Natl. Acad. Sci. USA 1993, 90, 9031–9035.
- 33 J. L. Sussman, M. Harel, F. Frolow, C. Oefner, A. Goldman, L. Toker, I. Silman, Science 1991, 253, 872–879.
- 34 H. A. Berman, M. Baker, M. McCauley, K. J. Leonard, M. W. Nowak, M. M. Decker, P. Taylor, Mol. Pharmacol. 1987, 31, 610–616.
- 35 A. S. Hodge, D. R. Humphrey, T. L. Rosenberry, Mol. Pharmacol. 1992, 41, 937–942.
- 36 Y.-P. Pang, P. Quiram, T. Jelacic, F. Hong, S. Brimijoin, J. Biol. Chem. 1996, 271, 23 646–23 649.
- 37
P. R. Carlier, D.-M. Du, Y.-F. Han, J. Liu, E. Perola, I. D. Williams, Y.-P. Pang, Angew. Chem. 2000, 112, 1845–1847;
10.1002/(SICI)1521-3757(20000515)112:10<1845::AID-ANGE1845>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1775–1777.10.1002/(SICI)1521-3773(20000515)39:10<1775::AID-ANIE1775>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 38 Z. Radić, P. Taylor, J. Biol. Chem. 2001, 7, 4622–4633.
- 39 See Supporting Information for details.
- 40 P. R. Carlier, Y. F. Han, E. S.-H. Chow, C. P.-L. Li, H.-S. Wang, T. X. Lieu, H. S. Wong, Y.-P. Pang, Bioorg. Med. Chem. 1999, 7, 351–357.
- 41 S. Lappi, P. Taylor, Biochemistry 1975, 14, 1989–1997.
- 42 S. A. Ross, M. Pitié, B. Meunier, J. Chem. Soc. Perkin Trans. 1 2000, 571–574.
- 43 The proposed structures were first examined computationally against AChE from electric ray Torpedo californica (PDB codes 1ACJ and 1ACL) with AutoDock 3.05[56] to ensure that tethers likely to be optimal would not be omitted. These calculations predicted that a chain length of 2–3 methylenes on the tacrine moiety and 5–6 on the phenanthridinium moiety would present favorable conformations to allow the cycloaddition reaction to occur in the enzyme.
- 44 E. Albert, F. Phillip in Alzheimer Disease: From Molecular Biology to Therapy ( ), Birkhauser, Boston, 1996, pp. 211–215.
- 45 Phenanthridinium/phenanthridinium combinations were not explored as they would not be expected to provide a dual-site inhibitor, the phenanthridinium moiety being too large to function as an active center ligand.
- 46 The tacrine components in water were each added to solutions of Electrophorus AChE (Sigma) in 2 mM ammonium citrate buffer (pH 7.3–7.5) and allowed to stand for 90 minutes at room temperature. The corresponding coupling partner was then added and the solutions were thoroughly mixed. The final concentrations were: AChE, 1.0 mg mL−1 of the commercial material (titrated concentration of functioning active centers=1 μM); tacrine component, 30 μM; phenanthridinium component, 66 μM.
- 47 A second-order rate constant of 1.9±0.7×10−5 M−1 min−1 was determined for the uncatalyzed reaction of TZ2 and PA6 over 9 days at 18 °C in 1-butanol. This is comparable to 7.0×10−5 M−1 min−1 obtained by Mock et al. at 40 °C.[26,27] Under these conditions, the reaction would take approximately 40 years to reach 80 % completion.
- 48 DIOS=Desorption/Ionization on Silicon.
- 49 DIOS-MS is uniquely tolerant of macromolecular impurities and buffer salts which allows convenient analysis of the crude reaction mixtures. J. Wei, J. Buriak, G. Siuzdak, Nature 1999, 401, 243–246; J. J. Thomas, Z. Shen, J. E. Crowell, M. G. Finn, G. Siuzdak, Proc. Natl. Acad. Sci. USA 2001, 98, 4932–4937; Z. Shen, J. J. Thomas, C. Averbuj, K. M. Broo, M. Engelhard, J. E. Crowell, M. G. Finn, G. Siuzdak, Anal. Chem. 2001, 73, 612–619.
- 50 Z. Radić, P. D. Kirchhoff, D. M. Quinn, J. A. McCammon, P. Taylor, J. Biol. Chem. 1997, 272, 23 265–23 277.
- 51 L. G. Ellman, K. D. Courtney, V. J. Andres, R. M. Featherstone, Biochem. Pharmacol. 1961, 7, 88–95.
- 52 The association rate constants (kon) were determined by both direct measurements of inhibitor binding to AChE and by measurements of time-dependent loss of AChE activity in reaction with inhibitor. The stopped-flow technique was used to measure rates of quenching of intrinsic AChE tryptophan fluorescence upon binding of inhibitor at micromolar concentrations as previously described.[38] The time-dependent loss of AChE activity was measured upon mixing AChE with picomolar concentrations of inhibitor in ten-fold excess. The AChE activity in aliquots of the reaction mixture was determined by Ellman assay[51] at intervals of several minutes to an hour. The second-order rate constants of inhibitor association were obtained by linear fit of first-order decay rates of either AChE fluorescence or its activity, at several inhibitor concentrations. The first-order dissociation constants (koff) were determined by measurements of the return of AChE activity by Ellman assay upon 5000-fold dilution of 50–100 nM concentrations of AChE⋅1 complex into 250 μg mL−1 solution of herring sperm DNA (Boehringer) in buffer (thereby the reassociation of the inhibitor to AChE by virtue of the affinity of the phenanthridinium part of the molecule for duplex DNA is competitively suppressed). The dissociation constant was determined by nonlinear fit of first-order increase in enzyme activity up to 70–80 % of the AChE control activity in a mixture containing no inhibitor. All experiments were performed in at least triplicate with the standard error of determination equal to or smaller than 20 % of the mean value. The measurements were performed in 0.1 M phosphate buffer pH 7.0 at 22 °C on a SX.18 MV stopped-flow instrument (Applied Photophysics) or Cary 1E UV/Vis spectrophotometer (Varian).
- 53 The trifluoroketone of Quinn and co-workers forms a hemiketal with the serine residue of the active center and exhibits a Ki value based on the total concentration of inhibitor of 80 pM. Most of this species exists in the inactive hydrated form. When one factors in the equilibrium concentration of the active carbonyl species, the inhibitor constant reaches low femtomolar levels [H. K. Nair, K. Lee, D. M. Quinn, J. Am. Chem. Soc. 1993, 115, 9939–9941; M. Harel, D. M. Quinn, H. K. Nair, J. Am. Chem. Soc. 1996, 118, 2340–2346]. Triazole syn-1 suffers no such equilibrium deactivation.
- 54 The inhibitory power of 1 exceeds even that of the fasciculin family of snake venom toxins, which occlude the mouth of the AChE gorge with binding constants of 0.44–40 pM, depending on the fasciculin and the source of the enzyme: P. Marchot, A. Khelif, Y. H. Ji, P. Mansuelle, P. E. Bougis, J. Biol. Chem. 1993, 268, 12 458–12 467; R. Duran, C. Cerveñansky, F. Dajas, K. F. Tipton, Biochim. Biophys. Acta 1994, 1201, 381–388; Z. Radić, R. Duran, D. C. Vellom, Y. Li, C. Cervenansky, P. Taylor, J. Biol. Chem. 1994, 269, 11 233–11 239; J. Eastman, E. J. Wilson, C. Cerveñansky, T. L. Rosenberry, J. Biol. Chem. 1995, 270, 19 696–19 701; G. Puu, M. Koch, Biochem. Pharmacol. 1990, 40, 2209–2214.
- 55 P. Camps, B. Cusack, W. D. Mallender, R. El Achab, J. Morral, D. Muñoz-Torrero, T. L. Rosenberry, Mol. Pharmacol. 2000, 57, 409–417.
- 56
G. M. Morris, D. S. Goodsell, R. S. Halliday, R. Huey, W. E. Hart, R. K. Belew, A. J. Olson, J. Comput. Chem. 1998, 19, 1639–1662.
10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B CAS Web of Science® Google Scholar
- 57 Although the lead inhibitor 1 was assembled by Electrophorus electricus AChE, it has a similarly high affinity for the Torpedo californica enzyme. This observation suggests a high degree of functional similarity with respect to binding in the active center gorge for these two enzymes (see: S. Simon, J. Massoulié, J. Biol. Chem. 1997, 272, 33 045–33 055), and justifies our use of the crystallographically well-characterized (2.8 Å resolution) Torpedo enzyme for modeling studies. At the present time Electrophorus AChE has only been characterized to 4.2 Å resolution ( Y. Bourne, J. Grassi, P. E. Bourgis, P. Marchot, J. Biol Chem. 1999, 274, 30 370–30 376).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:6<>1.0.co;2-7.cover.gif)
