The First Water-Soluble Copper(I) Calix[6]arene Complex Presenting a Hydrophobic Ligand Binding Pocket: A Remarkable Model for Active Sites in Metalloenzymes
Yannick Rondelez
Laboratoire de Chimie et Biochimie Pharmacologiques et Toxicologiques CNRS UMR 8601, Université René Descartes 45 rue des Saints-Pères, 75270 Paris Cedex 06 (France) Fax: (+33) 1-42-86-83-87
Search for more papers by this authorGildas Bertho
Laboratoire de Chimie et Biochimie Pharmacologiques et Toxicologiques CNRS UMR 8601, Université René Descartes 45 rue des Saints-Pères, 75270 Paris Cedex 06 (France) Fax: (+33) 1-42-86-83-87
Search for more papers by this authorOlivia Reinaud
Laboratoire de Chimie et Biochimie Pharmacologiques et Toxicologiques CNRS UMR 8601, Université René Descartes 45 rue des Saints-Pères, 75270 Paris Cedex 06 (France) Fax: (+33) 1-42-86-83-87
Search for more papers by this authorYannick Rondelez
Laboratoire de Chimie et Biochimie Pharmacologiques et Toxicologiques CNRS UMR 8601, Université René Descartes 45 rue des Saints-Pères, 75270 Paris Cedex 06 (France) Fax: (+33) 1-42-86-83-87
Search for more papers by this authorGildas Bertho
Laboratoire de Chimie et Biochimie Pharmacologiques et Toxicologiques CNRS UMR 8601, Université René Descartes 45 rue des Saints-Pères, 75270 Paris Cedex 06 (France) Fax: (+33) 1-42-86-83-87
Search for more papers by this authorOlivia Reinaud
Laboratoire de Chimie et Biochimie Pharmacologiques et Toxicologiques CNRS UMR 8601, Université René Descartes 45 rue des Saints-Pères, 75270 Paris Cedex 06 (France) Fax: (+33) 1-42-86-83-87
Search for more papers by this authorWe would like to thank Dr. Michèle Salmain (ENSCP, Paris) for her help with the IR spectroscopy experiments performed in water, and express our gratitude to Dr. Olivier Laprevotte (ICSN, Gif-sur-Yvette) for electron spray mass spectra.
Graphical Abstract
Deutliche Stabilisierung erfährt ein Kupfer(i)-Zentrum im gezeigten Trisulfonatocalix[6]aren-Komplex, der die Bezeichnung „biomimetisch“ wirklich verdient, da er wasserlöslich ist und eine freie Bindungsstelle für z. B. CO aufweist. Damit imitiert er die erste Koordinationssphäre von Kupferenzymen und die hydrophobe Mikroumgebung des aktiven Zentrums.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.com or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
S. J. Lippard, J. M. Berg, Principles of Bioinorganic Chemistry, University Science Books, Mill Valley, California 1994;
D. E. Fenton, Chem. Soc. Rev. 1999, 28, 159–168; reviews on bioinorganic enzymology:
Chem. Rev. 1996, 96, 2237–3042; for a review on copper bioinorganic chemistry, see:
W. Kaim, J. Rall, Angew. Chem. 1996, 108, 47–67;
10.1002/ange.19961080105 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 43–60; for recent examples on copper enzymes modeling, see: H. Decker, R. Dillinger, F. Tuczek, Angew. Chem. 2000, 112, 1656–1660;10.1002/(SICI)1521-3757(20000502)112:9<1656::AID-ANGE1656>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. Engl. 2000, 39, 1591–1595;10.1002/(SICI)1521-3773(20000502)39:9<1591::AID-ANIE1591>3.0.CO;2-H CAS PubMed Web of Science® Google ScholarP. Chen, K. Fujisawa, E. I. Solomon, J. Am. Chem. Soc. 2000, 122, 10 177–10 193; I. Blain, M. Giorgi, I. de Riggi, M. Réglier, Eur. J. Inorg. Chem. 2001, 205–211.
- 2
S. Trofimenko, Scorpionates, Imperial College Press, London, 1999.
10.1142/p148 Google Scholar
- 3
W. Kläui, M. Berghalm, G. Rheinwald, H. Lang, Angew. Chem. 2000, 112, 2590–2592;
10.1002/1521-3757(20000717)112:14<2590::AID-ANGE2590>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2464–2466.10.1002/1521-3773(20000717)39:14<2464::AID-ANIE2464>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 4 N. Psychologios, J.-B. Regnouf-de-Vains, Tetrahedron Lett. 2001, 42, 2799–2800; F. Tisato, F. Refosco, G. Bandoli, G. Pilloni, B. Corain, Inorg. Chem. 2001, 40, 1394–1396, and references therein.
- 5
- 5a
S. Blanchard, L. Le Clainche, M.-N. Rager, B. Chansou, J.-P. Tuchagues, A. F. Duprat, Y. L. Mest, O. Reinaud, Angew. Chem. 1998, 110, 2861–2864;
10.1002/(SICI)1521-3757(19981002)110:19<2861::AID-ANGE2861>3.0.CO;2-8 Google ScholarAngew. Chem. Int. Ed. Engl. 1998, 37, 2732–2735;10.1002/(SICI)1521-3773(19981016)37:19<2732::AID-ANIE2732>3.0.CO;2-7 CAS Web of Science® Google Scholar
- 5b
Y. Rondelez, O. Sénèque, M.-N. Rager, A. Duprat, O. Reinaud, Chem. Eur. J. 2000, 6, 4218–4226. This supramolecular system also allowed the stabilization of the first [N3ZnOH2]2+ complex, mimicking thereby the active site of zinc enzymes; see:
10.1002/1521-3765(20001117)6:22<4218::AID-CHEM4218>3.0.CO;2-V CAS PubMed Web of Science® Google ScholarO. Sénèque, M.-N. Rager, M. Giorgi, O. Reinaud, J. Am. Chem. Soc. 2001, 123, 8442–8443.
- 6 Experimental procedures and characterizations of all new compounds are provided in the Supporting Information.
- 7 Cupric complexes are not discussed here; however, studies related to the organic-solvent soluble calixarene-based system also showed strong similarities with the type 2 cupric centers of enzymes. See: L. Le Clainche, M. Giorgi, O. Reinaud, Inorg. Chem. 2000, 39, 3436–3437, and ref. [16].
- 8 A. Casnati, L. Domiano, A. Pochini, R. Ungaro, M. Carramolino, J. O. Magrans, P. M. Nieto, J. Lopez-Prados, P. Prados, J. de Mendoza, R. G. Janssen, W. Verboom, D. N. Reinhoudt, Tetrahedron 1995, 51, 12 699–12 720.
- 9 Although we cannot definitely exclude the presence of water as a fourth ligand on the metal ion, spectroscopic data were more consistent with a complex with the formula [3⋅Cu]2−, in which the cuprous ion is only tricoordinate. Indeed, the 1H NMR shifts were almost unaffected by the addition of an alcohol (methanol or ethanol) into D2O. In the IR spectrum no vibration attesting to a hydroxylic ligand could be detected. Finally, the electron spray mass spectrum of this complex in a water/methanol mixture only showed a peak corresponding to [3⋅Cu]2−, in which no guest molecule is present. For a recent example of a tricoordinate imidazolyl CuI complex, see: J. K. Voo, K. C. Lam, A. L. Rheingold, C. G. Riordan, J. Chem. Soc. Dalton Trans. 2001, 1803–1805.
- 10 This is 20–30 cm−1 below the νCO observed for the analogue soluble in organic solvents. This shift may be due to the small geometric differences (see ref. [4] and [11]).
- 11 The C3 symmetry is also compatible with an 1,3,5-alternate conformation. Despite the fact that this conformation has been envisaged in numerous papers, it has never been retained on the basis of structural analysis.
- 12
The Δδ shifts for Ha vs Hd and Hb vs He are 1.09 and 1.01, respectively. This is much higher than the shift by ca. 0.5 expected for a simple electronic effect due to the bromo and sulfonato substituents. For para-substituted toluenes the ortho protons (relative to Br or SO
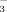 ) have chemical shifts of δ=7.35 (CDCl3) and 7.85 (D2O), respectively. Therefore, we can assume that the largest Δδ observed for the CuCO complex reflects conformational effects with HAr being in either in or out position relative to the aromatic walls of the calixarene cone (see ref. [11]).
) have chemical shifts of δ=7.35 (CDCl3) and 7.85 (D2O), respectively. Therefore, we can assume that the largest Δδ observed for the CuCO complex reflects conformational effects with HAr being in either in or out position relative to the aromatic walls of the calixarene cone (see ref. [11]).
- 13 Among the tert-butylated organic-solvent soluble analogues, the complexes bearing three O-methyl groups were shown to include one of their tBu moieties into the calixarene pocket which lead to a dissymetric conformation. This move is obviously disfavored by the replacement of tBu groups by sulfonates. Hence, the overall properties the water-soluble CuCO complex are more reminiscent of those reported for the organic-solvent soluble parent compound bearing three O-ethyl substituents (see ref. [5b]). However, the relative alternate position of the phenoxy walls is now reversed and corresponds to that depicted for the CuI-nitrilo complex in ref. [5a]. This may be due to the hydrophobic effect, which pushes the methoxy groups toward the cavity away from water.
- 14 J. L. Atwood, D. L. Clark, R. K. Juneja, G. W. Orr, K. D. Robinson, R. L. Vincent, J. Am. Chem. Soc. 1992, 114, 755; R. Castro, L. A. Godinez, C. M. Criss, S. M. Bott, A. E. Kaifer, Chem. Commun. 1997, 935–936; R. Castro, L. A. Godinez, C. M. Criss, A. E. Kaifer, J. Org. Chem. 1997, 62, 4928–4935; J. Alvarez, Y. Wang, M. Gomez-Kaifer, A. E. Kaifer, Chem. Commun. 1998, 1455–1456.
- 15 O. Sénèque, M.-N. Rager, M. Giorgi, O. Reinaud, J. Am. Chem. Soc. 2000, 122, 6183–6189.
- 16 L. Le Clainche, Y. Rondelez, O. Sénèque, S. Blanchard, M. Campion, M. Giorgi, A. F. Duprat, Y. L. Mest, O. Reinaud, C. R. Acad. Sci. Ser. IIc 2000, 3, 811–819.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:6<>1.0.co;2-7.cover.gif)
