Highly Regio- and Enantioselective Reduction of 3,5-Dioxocarboxylates
Michael Wolberg Dipl.-Chem.
Institut für Biotechnologie 2 Forschungszentrum Jülich GmbH 52425 Jülich (Germany) Fax (+49) 2461-61-3870
Search for more papers by this authorWerner Hummel Dr.
Institut für Enzymtechnologie Heinrich-Heine-Universität Düsseldorf 40225 Düsseldorf (Germany)
Search for more papers by this authorChristian Wandrey Prof. Dr.
Institut für Biotechnologie 2 Forschungszentrum Jülich GmbH 52425 Jülich (Germany) Fax (+49) 2461-61-3870
Search for more papers by this authorMichael Müller Dr.
Institut für Biotechnologie 2 Forschungszentrum Jülich GmbH 52425 Jülich (Germany) Fax (+49) 2461-61-3870
Search for more papers by this authorMichael Wolberg Dipl.-Chem.
Institut für Biotechnologie 2 Forschungszentrum Jülich GmbH 52425 Jülich (Germany) Fax (+49) 2461-61-3870
Search for more papers by this authorWerner Hummel Dr.
Institut für Enzymtechnologie Heinrich-Heine-Universität Düsseldorf 40225 Düsseldorf (Germany)
Search for more papers by this authorChristian Wandrey Prof. Dr.
Institut für Biotechnologie 2 Forschungszentrum Jülich GmbH 52425 Jülich (Germany) Fax (+49) 2461-61-3870
Search for more papers by this authorMichael Müller Dr.
Institut für Biotechnologie 2 Forschungszentrum Jülich GmbH 52425 Jülich (Germany) Fax (+49) 2461-61-3870
Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft in the scope of SFB 380. We thank Silke Bode for skillful technical support.
Abstract
Alkoholdehydrogenase aus Lactobacillus brevis (LBADH) katalysiert hochselektiv die Reduktion von 3,5-Dioxocarboxylaten in Position C-5 (siehe Schema). In Anlehnung an die natürlichen Transformationen des Polyketidstoffwechsels wurde ein chemoenzymatischer Zugang zu nahezu enantiomerenreinen 3,5-Dihydroxycarboxylaten entwickelt. Der entscheidende enzymatische Schritt kann in einem präparativ attraktiven Maßstab durchgeführt werden.
References
- 1a D. J. Ager, S. A. Laneman, Tetrahedron: Asymmetry 1997, 8, 3327–3355;
- 1b H. Brunner, Methods of Organic Chemistry (Houben-Weyl) 4th ed., Vol. E21d, 1995, pp. 3957–3964.
- 2a R. Csuk, B. I. Glänzer in Stereoselective Biocatalysis ( ), Marcel Dekker, New York, 2000, p. 544;
- 2b P. D'Arrigo, G. Pedrocchi-Fantoni, S. Servi in Stereoselective Biocatalysis ( ), Marcel Dekker, New York, 2000, p. 373;
- 2c M. Gottwald, Methods of Organic Chemistry (Houben-Weyl) 4th ed., Vol. E21d, 1995, pp. 4143–4157; M. Gottwald, Methods of Organic Chemistry (Houben-Weyl) 4th ed., Vol. E21d, 1995, pp. 4167–4169.
- 3a
V. Blandin, J.-F. Carpentier, A. Mortreux, Eur. J. Org. Chem. 1999, 3421–3427;
10.1002/(SICI)1099-0690(199912)1999:12<3421::AID-EJOC3421>3.0.CO;2-1 CAS Web of Science® Google Scholar
- 3b L. Shao, H. Kawano, M. Saburi, Y. Uchida, Tetrahedron 1993, 49, 1997–2010;
- 3c L. Shao, T. Seki, H. Kawano, M. Saburi, Tetrahedron Lett. 1991, 32, 7699–7702;
- 3d N. Sayo, T. Saito, H. Kumobayashi, S. Akutagawa, R. Noyori, H. Takaya Takasago International Corp., EP-A 297 752, 1989 [ Chem. Abstr. 1989, 111, 114 745n].
- 4a
M.-R. Kula, U. Kragl in Stereoselective Biocatalysis ( ), Marcel Dekker, New York, 2000, pp. 839–866;
10.1201/9781420027242.ch28 Google Scholar
- 4b J. Peters in Biotechnology, Vol. 8a ( ), 2nd ed., Wiley-VCH, Weinheim, 1998, pp. 391–474;
- 4c W. Hummel in Advances in Biochemical Engineering/Biotechnology, Vol. 58 ( ), Springer, Berlin, 1997, pp. 145–184;
- 4d R. Devaux-Basseguy, A. Bergel, M. Comtat, Enzyme Microb. Technol. 1997, 20, 248–258.
- 5a R. N. Patel, A. Banerjee, C. G. McNamee, D. Brzozowski, R. L. Hanson, L. J. Szarka, Enzyme Microb. Technol. 1993, 15, 1014–1021;
- 5b R. N. Patel, C. G. McNamee, A. Banerjee, L. J. Szarka Squibb &Sons, Inc., EP-A 569 998, 1993 [ Chem. Abstr. 1994, 120, 52 826q];
- 5c M. Uko, H. Azuma, T. Sakai, S. Tsuboi Mitsubishi Kasei Corp., JP-Kokai 03 48 641, 1991 [ Chem Abstr. 1991, 115, 28 713b];
- 5d part of the present work was presented by M.M. at the 27th General Meeting of the Gesellschaft Deutscher Chemiker, Berlin, August 18, 1999 (abstract L-76).
- 6a K.-M. Chen, G. E. Hardtmann, K. Prasad, O. Repič, M. J. Shapiro, Tetrahedron Lett. 1987, 28, 155–158;
- 6b D. A. Evans, K. T. Chapman, E. M. Carreira, J. Am. Chem. Soc. 1988, 110, 3560–3578;
- 6c D. A. Evans, K. T. Chapman, Tetrahedron Lett. 1986, 27, 5939–3942.
- 7 Some examples from the literature: tetrahydrolipstatin:
- 7a S. Hanessian, A. Tehim, P. Chen, J. Org. Chem. 1993, 58, 7768–7781;
- 7b P. Barbier, F. Schneider, J. Org. Chem. 1988, 53, 1218–1221; bryostatin 2:
- 7c
D. A. Evans, P. H. Carter, E. M. Carreira, A. B. Charette, J. A. Prunet, M. Lautens, Angew. Chem. 1998, 110, 2526–2530; Angew. Chem. Int. Ed. 1998, 37, 2354–2359; alternaric acid:
10.1002/(SICI)1521-3757(19980904)110:17<2526::AID-ANGE2526>3.0.CO;2-B Web of Science® Google Scholar
- 7d H. Tabuchi, T. Hamamoto, S. Miki, T. Tejima, A. Ichihara, J. Org. Chem. 1994, 59, 4749–4759; tarchonanthus lactone:
- 7e G. Solladié, L. Gressot-Kempf, Tetrahedron: Asymmetry 1996, 7, 2371–2379; HMG CoA reductase inhibitors:
- 7f G. Beck, H. Jendralla, K. Kesseler, Synthesis 1995, 1014–1018;
- 7g J. K. Thottathil, Y. Pendri, W. S. Li, D. R. Kronenthal Squibb & Sons, Inc., US 5 278 313, 1994 [ Chem. Abstr. 1994, 120, 217 699r].
- 8 B. Riebel, Dissertation, Universiät Düsseldorf, 1996. A 10-L fermentation of E. coli strain recADH-HB101+ yields approximately 600 000 U of recLBADH.
- 9 Prepared according to F. Yuste, F. K. Breña, H. Barrios, R. Sánchez-Obregón, B. Ortiz, F. Walls, Synth. Commun. 1988, 18, 735–739. Other alkyl esters of 3,5-dioxohexanoic acid can be used in the enzymatic reaction as well.
- 10 P.-F. Deschenaux, T. Kallimopoulos, H. Stoeckli-Evans, A. Jacot-Guillarmod, Helv. Chim. Acta 1989, 72, 731–737.
- 11 D. Drochner, M. Müller, Eur. J. Org. Chem., in press.
- 12 DAICEL Chiracel OB (plus guard column), isohexane/2-propanol (80/20 v/v), 1.0 mL min−1, 25 °C, 215 nm (UV-DAD). Retention times: (S)-3: 17.6, (R)-3: 23.8, (R)-6: 20.9, (S)-6: 24.3 min. In the case of the (S)-lactone derived from the enzymatic product 5, none of the R enantiomer could be detected.
- 13 Prepared by acylation of the lithium bis-enolate of tert-butyl acetoacetate with methyl chloroacetate (THF, −60 °C, 70 % yield of isolated product after column chromatography).
- 14
Prepared according to ref. [7g], starting from commercially available ethyl (S)-4-chloro-3-hydroxybutyrate (Aldrich, 97 % ee). [α]
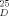 =−23.0 (c=1.5, CHCl3), 97 % ee; enzymatic product 5: [α]
=−23.0 (c=1.5, CHCl3), 97 % ee; enzymatic product 5: [α]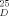 =−24.9 (c=1.4, CHCl3), >99.5 % ee; 1H NMR (300 MHz, CDCl3, 20 °C, only signals of the keto form are given): δ=4.31 (m, 1 H; CHOH), 3.62 (dd, J=11.2, 5.1 Hz, 1 H; H-6), 3.57 (dd, J=11.2, 5.0 Hz, 1 H; H-6), 3.41 (s, 2 H; H-2), 3.10 (br s, 1 H; OH), 2.90 (dd, J=17.5, 5.0 Hz, 1 H; H-4), 2.83 (dd, J=17.5, 7.3 Hz, 1 H; H-4), 1.47 (s, 9 H, C(CH3)3); 13C NMR (75.5 MHz, CDCl3, 20 °C, only signals of the keto form are given): δ=28.1 (C(CH3)3), 46.6, 48.4, 51.3 (C-2, C-4, C-6), 67.6 (C-5), 82.7 (C(CH3)3), 166.2 (C-1), 202.9 (C-3).
=−24.9 (c=1.4, CHCl3), >99.5 % ee; 1H NMR (300 MHz, CDCl3, 20 °C, only signals of the keto form are given): δ=4.31 (m, 1 H; CHOH), 3.62 (dd, J=11.2, 5.1 Hz, 1 H; H-6), 3.57 (dd, J=11.2, 5.0 Hz, 1 H; H-6), 3.41 (s, 2 H; H-2), 3.10 (br s, 1 H; OH), 2.90 (dd, J=17.5, 5.0 Hz, 1 H; H-4), 2.83 (dd, J=17.5, 7.3 Hz, 1 H; H-4), 1.47 (s, 9 H, C(CH3)3); 13C NMR (75.5 MHz, CDCl3, 20 °C, only signals of the keto form are given): δ=28.1 (C(CH3)3), 46.6, 48.4, 51.3 (C-2, C-4, C-6), 67.6 (C-5), 82.7 (C(CH3)3), 166.2 (C-1), 202.9 (C-3).
- 15 The diastereomeric excess was determined by GC after formation of the corresponding acetonides (2,2-dimethoxypropane, cat. camphorsulfonic acid).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



112:23<>1.0.co;2-b.cover.gif)
