Alumoxane Hydride and Aluminum Chalcogenide Hydride Compounds with Pyrazolato Ligands
Wenjun Zheng Dipl.-Chem.
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorNadia C. Mösch-Zanetti Dr.
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorHerbert W. Roesky Prof. Dr.
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorMathias Noltemeyer Dr.
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorManuel Hewitt
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorHans-Georg Schmidt
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorThomas R. Schneider Dr.
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorWenjun Zheng Dipl.-Chem.
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorNadia C. Mösch-Zanetti Dr.
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorHerbert W. Roesky Prof. Dr.
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorMathias Noltemeyer Dr.
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorManuel Hewitt
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorHans-Georg Schmidt
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorThomas R. Schneider Dr.
Institut für Anorganische Chemie der Universität Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft. N.C.M.-Z. thanks the Schweizerischer Nationalfonds for a fellowship and W.Z. thanks Dr. U. Ritter for the GC analysis.
Abstract
Zwei fünffach koordinierte Aluminiumatome enthält der planare Al2O2-Kern in 1, der zudem ungewöhnlich kurze Al-O-Bindungen aufweist. 1 wurde aus einem Aluminiumdihydrid-Komplex und Dioxan synthetisiert. Aus dem Edukt können durch Reaktionen mit S, Se und Te weitere chalkogenverbrückte Aluminiumhydride entstehen.
References
- 1a H. Sinn, W. Kaminsky, H.-J. Vollmer, R. Woldt, Angew. Chem. 1980, 92, 396; Angew. Chem. Int. Ed. Engl. 1980, 19, 390;
- 1b H. Sinn, W. Kaminsky, Adv. Organomet. Chem. 1980, 18, 99.
- 2 Alkylalumoxanes generally contain an oxygen bridge between two metal atoms (Al-O-Al). Oligomeric aluminum alkoxides (aluminum hydroxides) bridged by an alkoxy (hydroxide) group (Al-O(R)-Al, Al-O(H)-Al) and compounds containing no organic group are usually not included in this classification: S. Pasynkiewicz, Polyhedron 1990, 9, 429.
- 3 Alkylalumoxanes are usually prepared by hydrolysis of organoaluminum compounds with water[3a] or hydrated metal salts,[3b] or upon reaction with species containing reactive oxygen such as CO2, RCONR2, MeCO2H, and Me2SO.[3c]
- 3a S. I. Ishida, J. Polym. Sci. 1962, 62, 1;
- 3b G. A. Razuvaev, Yu. A. Sangalov, Yu. Ya. Ne'lkenbaum, K. S. Minsker, Izv. Akad. Nauk SSSR. Ser. Khim. 1975, 2547 [ Chem. Abstr. 1976, 84, 59627x]; S. Collins, W. J. Gauthier, D. A. Holden, B. A. Kuntz, N. J. Taylor, D. G. Ward, Organometallics 1991, 10, 2061;
- 3c K. Ziegler, F. Krupp, K. Weyer, W. Larbig, Justus Liebigs Ann. Chem. 1960, 629, 251; L. J. Zakharkin, I. M. Khorlina, Izv. Akad. Nauk SSSR. Ser. Khim. 1959, 2146.
- 4
H.-H. Brintzinger, D. Fischer, R. Mühlhaupt, B. Rieger, R. Waymouth, Angew. Chem. 1995, 107, 1255; Angew. Chem. Int. Ed. Engl. 1995, 34, 1143, and references therein.
10.1002/ange.19951071104 Google Scholar
- 5a M. R. Mason, J. M. Smith, S. G. Bott, A. R. Barron, J. Am. Chem. Soc. 1993, 115, 4971;
- 5b C. J. Harlan, M. R. Mason, A. R. Barron, Organometallics 1994, 13, 2957;
- 5c
C. C. Landry, C. J. Harlan, S. G. Bott, A. R. Barron, Angew. Chem. 1995, 107, 1315; Angew. Chem. Int. Ed. Engl. 1995, 107, 1201.
10.1002/ange.19951071110 Google Scholar
- 6a J. Storre, A. Klemp, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, R. Fleischer, D. Stalke, J. Am. Chem. Soc. 1996, 118, 1380;
- 6b J. Storre, C. Schnitter, H. W. Roesky, H.-G. Schmidt, M. Noltemeyer, R. Fleischer, D. Stalke, J. Am. Chem. Soc. 1997, 119, 7505.
- 7 R. J. Wehmschulte, P. P. Power, J. Am. Chem. Soc. 1997, 119, 8387.
- 8a R. J. Wehmschulte, P. P. Power, J. Am. Chem. Soc. 1997, 119, 9566;
- 8b R. J. Wehmschulte, P. P. Power, Chem. Commun. 1998, 335.
- 9a M. G. Gardiner, C. L. Raston, V.-A. Tolhurst, J. Chem. Soc. Chem. Commun. 1995, 1457;
- 9b W. J. Grigsby, C. L. Raston, V. A. Talhurst, B. W. Skelton, A. H. White, J. Chem. Soc. Dalton Trans. 1998, 2549.
- 10a C. Cui, H. W. Roesky, M. Noltemeyer, H.-G. Schmidt, Organometallics 1999, 18, 5120;
- 10b
C. Cui, H. W. Roesky, H. Hao, H.-G. Schmidt, M. Noltemeyer, Angew. Chem. 2000, 112, 1885; Angew. Chem. Int. Ed. 2000, 39, 1815.
10.1002/(SICI)1521-3757(20000515)112:10<1885::AID-ANGE1885>3.0.CO;2-K Web of Science® Google Scholar
- 11 W. Zheng, N. C. Mösch-Zanetti, H. W. Roesky, M. Hewitt, F. Cimpoesu, T. R. Schneider, A. Stasch, J. Prust, Angew. Chem. 2000, 112, 3229; Angew. Chem. Int. Ed. 2000, 39, 3099.
- 12
X-ray crystal structure determination: The data for 2–5 were collected on a Stoe-Siemens four-circle diffractometer (at −70(2) °C) with graphite-monochromated MoKα radiation (λ=0.71073 Å). The structures were solved by direct methods and refined against F 2 on all data by full-matrix least squares with SHELXS-97.[16] 2 (C56H104Al4N8O8; includes three dioxane molecules): Mr=1125.39, triclinic, space group
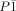 , a=10.202(5), b=13.128(6), c=13.612(5) Å, α=112.39(3), β=101.90(2), γ=96.936(13)°, V=1608.7(12) Å3, Z=1, ρcalcd=1.162 Mg m−3, F(000)=612, μ(MoKα)=0.127 mm−1. A total of 9401 reflections were measured in the range 7.20≤2θ≤50.08°, of which 5673 were unique. Final R indices: R1=0.0404 (I>2σ(I)), wR2=0.1113 (all data); max./min. residual electron density 307/−253 e nm−3. 3 (C22H40Al2N4S): Mr=446.6, monoclinic, space group P21/n, a=14.407(2), b=11.0570(14), c=16.506(3) Å, β=98.54(2)°, V=2600.1(8) Å3, Z=4, ρcalcd=1.141 Mg m−3, F(000)=968, μ(MoKα)=0.207 mm−1. A total of 9761 reflections were measured in the range 7.02≤2θ≤50.06°, of which 4581 were unique. Final R indices: R1=0.0301 (I>2σ(I)), wR2=0.0870 (all data); max./min. residual electron density 263/−183 e nm−3. 4 (C22H40Al2N4Se): Mr=494, monoclinic, space group P21/n, a=14.385(3), b=11.035(2), c=16.522(3) Å, β=98.90(3)°, V=2591.1(9) Å3, Z=6, ρcalcd=1.265 Mg m−3, F(000)=1040, μ(MoKα)=1.532 mm−1. A total of 5301 reflections were measured in the range 4.46≤2θ≤52.74°, of which 5301 were unique. Final R indices: R1=0.0415 (I>2σ(I)), wR2=0.1474 (all data); max./min. residual electron density 551/−944 e nm−3. 5 (C29H48Al2N4Te; includes one toluene molecule): Mr=634.27, monoclinic, space group P21/n, a=12.288(2), b=15.592(3), c=18.031(3) Å, β=106.295(14)°, V=3316.1(9) Å3, Z=4, ρcalcd=1.270 Mg m−3, F(000)=1312, μ(MoKα)=0.972 mm−1. A total of 7932 reflections were measured in the range 7.02≤2θ≤50.04°, of which 5847 were unique. Final R indices: R1=0.0389 (I>2σ(I)), wR2=0.0991 (all data); max./min. residual electron density 1006/−694 e nm−3. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC-145888 (2), CCDC-145889 (3), CCDC-145981 (4), and CCDC-145982 (5). Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
, a=10.202(5), b=13.128(6), c=13.612(5) Å, α=112.39(3), β=101.90(2), γ=96.936(13)°, V=1608.7(12) Å3, Z=1, ρcalcd=1.162 Mg m−3, F(000)=612, μ(MoKα)=0.127 mm−1. A total of 9401 reflections were measured in the range 7.20≤2θ≤50.08°, of which 5673 were unique. Final R indices: R1=0.0404 (I>2σ(I)), wR2=0.1113 (all data); max./min. residual electron density 307/−253 e nm−3. 3 (C22H40Al2N4S): Mr=446.6, monoclinic, space group P21/n, a=14.407(2), b=11.0570(14), c=16.506(3) Å, β=98.54(2)°, V=2600.1(8) Å3, Z=4, ρcalcd=1.141 Mg m−3, F(000)=968, μ(MoKα)=0.207 mm−1. A total of 9761 reflections were measured in the range 7.02≤2θ≤50.06°, of which 4581 were unique. Final R indices: R1=0.0301 (I>2σ(I)), wR2=0.0870 (all data); max./min. residual electron density 263/−183 e nm−3. 4 (C22H40Al2N4Se): Mr=494, monoclinic, space group P21/n, a=14.385(3), b=11.035(2), c=16.522(3) Å, β=98.90(3)°, V=2591.1(9) Å3, Z=6, ρcalcd=1.265 Mg m−3, F(000)=1040, μ(MoKα)=1.532 mm−1. A total of 5301 reflections were measured in the range 4.46≤2θ≤52.74°, of which 5301 were unique. Final R indices: R1=0.0415 (I>2σ(I)), wR2=0.1474 (all data); max./min. residual electron density 551/−944 e nm−3. 5 (C29H48Al2N4Te; includes one toluene molecule): Mr=634.27, monoclinic, space group P21/n, a=12.288(2), b=15.592(3), c=18.031(3) Å, β=106.295(14)°, V=3316.1(9) Å3, Z=4, ρcalcd=1.270 Mg m−3, F(000)=1312, μ(MoKα)=0.972 mm−1. A total of 7932 reflections were measured in the range 7.02≤2θ≤50.04°, of which 5847 were unique. Final R indices: R1=0.0389 (I>2σ(I)), wR2=0.0991 (all data); max./min. residual electron density 1006/−694 e nm−3. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC-145888 (2), CCDC-145889 (3), CCDC-145981 (4), and CCDC-145982 (5). Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
- 13a S. J. Dzugan, V. L. Goedken, Inorg. Chem. 1986, 25, 2858;
- 13b J. A. Francis, S. G. Bott, A. R. Barron, J. Chem. Soc. Dalton Trans. 1998, 3305;
- 13c M. D. Healy, A. R. Barron, J. Am. Chem. Soc. 1989, 111, 398;
- 13d S. G. Bott, H. Elgamal, J. L. Atwood, J. Am. Chem. Soc. 1985, 107, 1796.
- 14 Recently, the electronic contributions of short Al−O bond lengths have received increased attention. However, observation of the shortening has given rise to controversy since several explanations for this feature are possible:
- 14a A. R. Barron, K. D. Dobbs, M. M. Francl, J. Am. Chem. Soc. 1991, 113, 39;
- 14b M. A. Petrie, M. M. Olmstead, P. P. Power, J. Am. Chem. Soc. 1991, 113, 8704;
- 14c
W. Uhl, M. Koch, W. Hiller, M. Heckel, Angew. Chem. 1995, 107, 1122; Angew. Chem. Int. Ed. Engl. 1995, 34, 989;
10.1002/ange.19951070927 Google Scholar
- 14d W. H. Fink, P. P. Power, T. L. Allen, Inorg. Chem. 1997, 36, 1431;
- 14e L. Boiteau, I. Demachy, F. Volatron, Chem. Eur. J. 1997, 3, 1860.
- 15 G. B. Deacon, E. E. Dilbridge, C. M. Forsyth, P. C. Junk, B. W. Skelton, A. H. White, Austr. J. Chem. 1999, 52, 733.
- 16 G. M. Sheldrick, SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Germany, 1997.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



112:23<>1.0.co;2-b.cover.gif)
