Bioprospecting the Rodriguan Lime (Citrus aurantifolia Swingle) as a Novel Source of Antioxidants and Antimicrobials for Food Application
Abstract
In view of bioprospecting the Rodriguan lime (Citrus aurantifolia Swingle) as a novel antioxidant and antimicrobial for the food industry, its bioactivities were compared with those of the Mauritian pamplemousses (Citrus maxima) and the Rodriguan grapefruit (Citrus × paradisi Macfad). The Rodriguan lime, “Limon Rodrigues,” is also known as the Mexican lime (Citrus aurantiifolia, Swingle) or key lime. All citrus peel extracts tested in the study—namely, the Rodriguan lime, Mauritian pamplemousses, and Rodriguan grapefruit—exhibited comparable antioxidant activity in the ferric reducing antioxidant power (FRAP) (14.50 ± 3.11, 12.96 ± 0.97, and 14.77 ± 1.47) and CUPRAC (cupric reducing antioxidant capacity) (0.71 ± 0.20, 0.50 ± 0.04, and 0.59 ± 0.11) assays. The Rodriguan lime extract had the lowest overall minimum inhibitory concentration (MIC) of 5–10 mg/mL against Staphylococcus aureus, Salmonella enterica, Listeria monocytogenes, Bacillus cereus, and Lactobacillus plantarum. Although the Rodriguan grapefruit peel had the highest total phenolic content (64.53 ± 3.25 mg GAE/g extract) (p < 0.05), its total flavonoid content was not significantly different from that of the Rodriguan lime peel (p > 0.05). LC-MS data revealed that the Rodriguan grapefruit extract possessed the highest overall concentration of flavonoids (4821.1 mg RE/kg) and coumarins (13476 mg CE/kg), although the Rodriguan lime peel extract exhibited a relatively unique flavonoid and coumarin profile. Citrus flavonoids and coumarins exhibit diverse biological functions, including antidiabetic, antimicrobial, antifungal, hypotensive, antioxidant, carminative, antibacterial, larvicidal, antiviral, uricosuric, antiyeast, antihepatotoxic, and antimutagenic activities. Additionally, they demonstrate significant anticancer, cardiovascular-protective, and neuroprotective properties. These multifunctional bioactive compounds highlight the potential of citrus-derived substances in therapeutic and preventive health applications. Given its broad antimicrobial spectrum and diverse phytochemicals, the Rodriguan lime extract shows potential for applications in the functional food and nutraceutical industries.
1. Introduction
The genus Citrus spp., belonging to the family Rutaceae, is one of the most significant fruit crops globally. According to the Food and Agriculture Organization (FAO), global citrus production reached 143.8 million tons in 2019, with cultivation spanning over 140 countries, making citrus one of the most commercialized horticultural products [1]. Citrus fruits are categorized into sweet oranges, lemons/limes, mandarins (including clementines and tangerines), and grapefruits (including pummelos) [2]. Approximately 80% of citrus harvests are utilized in the juice industry, generating substantial by-products—comprising peels, seeds, and pulp—which represent up to 55% of the fruit’s weight. These by-products pose significant environmental disposal challenges, underscoring the necessity of value-added utilization strategies to mitigate their ecological impact [3].
Citrus by-products are rich in dietary fiber, minerals, and bioactive compounds, including vitamins B, ascorbic acid, coumarins, carotenoids, flavonoids, and essential oils (terpenes and limonoids) [2, 4]. The peel, which constitutes 50% of the fruit’s mass, is particularly abundant in phenolic compounds such as flavanones, flavonols, and polymethoxylated flavones, which are rare in other plant species [4]. Key flavonoids include hesperidin, naringin, narirutin, and eriocitrin [5, 6]. Additionally, coumarins and furanocoumarins, such as bergapten and isopimpinellin, have been identified in citrus peels, where they play roles in pathogen defense [7, 8]. These phytochemicals have been extensively studied for their physiological, ecological, and industrial applications, exhibiting a broad range of bioactivities including antioxidant, antimicrobial, antifungal, anti-inflammatory, and anticancer properties [6, 9, 10].
The Rodriguan lime (Citrus aurantifolia Swingle), also known as “Limon Rodrigues,” is the predominant citrus variety on Rodrigues, an island in the Republic of Mauritius. This species stands out due to its exceptional drought tolerance and ability to thrive in marginal growing conditions [11]. Unlike the common key lime, the Rodriguan lime has remained genetically isolated due to the island’s geographical separation, resulting in unique phytochemical properties [12]. Additionally, it reaches full maturity while retaining its distinctive green rind, making it competitive with limes from other regions where early yellowing occurs due to climate. Despite its ecological and agricultural advantages, the phytochemical composition and bioactivities of the Rodriguan lime remain largely unexplored, representing a significant research gap.
This study is the first to characterize the Rodriguan lime peel extract and compare its bioactive properties with those of Rodriguan grapefruit (Citrus × paradisi) and Mauritian pamplemousses (Citrus × maxima). The novelty of this work lies in demonstrating the Rodriguan lime’s unique phytochemical profile, exceptional drought tolerance, and novel antimicrobial properties. These features position the Rodriguan lime as an ideal candidate for value-added applications in functional foods and nutraceuticals. Furthermore, the study highlights its potential use in developing edible coatings enriched with natural antimicrobials and antioxidants. Such coatings could be applied to minimally processed and whole fresh fruits and vegetables, extending their shelf life while meeting the growing demand for clean-label, sustainable food preservation solutions. This approach aligns with global trends in reducing food waste, promoting sustainable agriculture, and enhancing food security.
2. Methodology
2.1. Collection of Fruits
The Rodriguan lime (Citrus aurantifolia Swingle) and Rodriguan grapefruit (Citrus × paradisi Macfad), which exhibited no signs of insect infestation or blemishes, were collected from Rodrigues Island, Mauritius (Figure 1). The Mauritian pamplemousses (Citrus maxima) was collected from Pamplemousses (20.1095° S, 57.5816° E), Mauritius, in 2021 and 2022.

2.2. Solvent Extraction and Preparation of Freeze-Dried Extracts
The fruits were washed with distilled water and peeled, and the peels were dried in an oven for 4 days at 65°C, then ground to a powder using a blender. A known quantity of each peel powder (2.5 g) was added into separate Erlenmeyer flasks containing 50 mL of 80% ethanol (v/v) and left to macerate for 48 h with occasional stirring [13]. The solvent was decanted, and fresh solvent was added to the residue, which was again left to macerate overnight. The extracts were pooled and filtered through sterile Whatman No. 1 filter paper and allowed to evaporate until dry. The condensed peel extracts were used for subsequent analysis (provide reference). Extractions were performed in triplicate.
Ethanol was removed from the crude peel extracts by rotary evaporation, and the crude extracts were then freeze-dried to remove excess moisture. The final freeze-dried crude extracts were in a hygroscopic powdered form and subsequently sealed in airtight containers and placed in frozen storage until needed for further analysis.
2.3. Characterisation of the Citrus Peel Extracts
2.3.1. Total Phenolic Content
The Folin–Ciocalteu method, as described by Nickavar and Esbati [14], was used to determine the total phenolic content. The reaction mixture contained 2500 μL of a 10-fold dilution of Folin–Ciocalteu reagent, 500 μL of the sample, and 2000 μL of sodium carbonate (7.5%). The reaction mixture was allowed to react for 30 min at room temperature. The sample’s total phenolic content was then measured at 760 nm using a spectrophotometer (Jenway Spectrophotometer 7305). The total phenolic content was measured in milligrams of gallic acid equivalent (GAE) per gram of extract after being computed using a gallic acid standard curve.
2.3.2. Total Flavonoid Content
The total flavonoid content was determined using the aluminum chloride colorimetric method described by Amaeze et al. [15]. Two milliliters of the sample and 2 mL of the 2% aluminum chloride solution made up the reaction mixture. After 30 min of reaction time at room temperature, the mixture’s absorbance was measured at 420 nm. Using a rutin standard curve, the total flavonoid content was calculated. The results were reported as milligrams of rutin equivalents (RE) per gram of extract.
2.3.3. Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP assay, as described by Benzie and Strain [16], was used to determine the samples’ ferric reducing antioxidant ability, with Trolox used as a standard. A total of 2850 μL of FRAP solution (25 mL hydrated ferric chloride solution [20 mM], 2.5 mL 2,4,6-tripyridyl-s-triazine [10 mM in 40 mM HCl], and acetate buffer [300 mM and pH 3.6]) were combined with the sample. After 30 min of dark incubation, the reaction mixture was measured for absorbance at 593 nm using a spectrophotometer (Jenway Spectrophotometer 7305). The results were reported as milligrams of Trolox equivalents (TE) per gram of crude extract.
2.3.4. Nitric Oxide (NO) Radical Scavenging Assay
By utilizing 0.1% w/v naphthylethylenedihydrochloride, NO was generated from sodium nitroprusside and quantified using the Griess reagent. A total of 50 μL of sodium nitroprusside (10 mM) and 50 μL of sample were added to the reaction mixture, which was then incubated at room temperature for 180 min. Following the incubation period, the mixture was mixed with 125 μL of Griess reagent and incubated for 10 min. Using a spectrophotometer (Jenway Spectrophotometer 7305), the absorbance was measured at 546 nm. The formula for calculating the inhibition percentage was %inhibition = [(negative control sample Abs)/negative control Abs] × 100. The IC50 was then determined using the % inhibition value.
2.3.5. Xanthine Oxidase (XO) Inhibition
In summary, 2.9 mL of phosphate buffer (pH 7.4), 0.1 mL of enzyme solution (0.04 units/mL), and 1 mL of the sample were combined, and the mixture was allowed to incubate at room temperature for 15 min. After adding 2 mL of xanthine solution (10 mM) and allowing the mixture to incubate at room temperature for 30 min, the reaction was initiated. One milliliter of HCl (1 N) was added to terminate the reaction, and a UV spectrophotometer (Jenway Spectrophotometer 7305) was used to detect absorbance at 290 nm.
2.3.6. Cupric Reducing Antioxidant Capacity (CUPRAC)
The method described by Grochowski et al. [17] was used to determine the CUPRAC of the samples. The peel extract (0.5 mL) was mixed with a reaction mixture containing CuCl2 (1 mL and 10 mM), neocuproine (1 mL and 7.5 mM), and NH4CH3CO2 buffer (1 mL, 1 M, and pH 7.0) and incubated at room temperature. The absorbance was measured at 450 nm using a spectrophotometer (Jenway Spectrophotometer 7305) after 30 min, and the results were expressed as milligrams TE per gram extract.
2.3.7. Identification and Quantification of Coumarins and Flavonoids Using Liquid Chromatography–Mass Spectrometry (LC-MS)
Phenolic compounds were analyzed by chromatographic analysis with a quenched ion mobility system as described by Stander et al. [18]. For coumarins, analysis was performed according to Dugo et al. [19]. Determination was carried out by comparing the chromatograms and retention times of the phenolic components in the extract with external standards of phenolic acids and flavonoids. Additionally, MS/MS fragmentation data and UV spectra were compared with those of phenolic compounds reported in the literature. Quantification was done by comparing integrated peak areas of phenolic compounds and coumarins in extracts with those of standards, namely, rutin and coumarin.
2.3.8. Determination of Antimicrobial Activity of the Citrus Extracts
Foodborne pathogens Staphylococcus aureus (ATCC 25923), Bacillus cereus (ATCC 14579), Salmonella enterica sv Typhimurium (ATCC 14028), Listeria monocytogenes (ATCC 13932), and Escherichia coli (ATCC 25922) and spoilage organisms Lactobacillus sp. (ATCC 15578), Issatchenkia orientalis (ATCC 6258), Penicillium citrinum (ATCC 9849), and Aspergillus niger (ATCC 1015) were used as test microorganisms. Cultures of bacteria were grown for 24 h in 10 mL of nutrient broth (HiMedia) at 37°C and were maintained on differential HiCrome agar (HiMedia) at 4°C.
2.3.8.1. Determination of Antimicrobial Effect Using Disc Diffusion Assay
The assessment of the antibacterial activity of natural extracts using the disc diffusion assay was conducted according to the standard method described by Bauer et al. [20]. The positive control used was chloramphenicol, a broad-spectrum antibiotic, prepared by dissolving 50 mg of chloramphenicol powder (HiMedia, India) in 60 μL of ethanol and 4.94 mL of distilled water to achieve a final concentration of 10 mg/mL. For the positive control, the different crude extracts were dissolved in 99% ethanol and distilled water to achieve a final concentration of 100 mg/mL. The negative or solvent control was prepared by mixing 60 μL of 99% ethanol (Supplies Solutions Ltd) with 4.94 mL of distilled water. Culti-Loops for each foodborne pathogen and spoilage organism were cultured on differential HiCrome media (HiMedia) and incubated for 24 h at 37°C. After incubation, a loopful of the colonies was transferred to 10 mL of nutrient broth (HiMedia) and incubated for another 24 h at 37°C. After 24 h, 1 mL of the broth was transferred to 9 mL of fresh nutrient broth for incubation at 37°C for another 24 h to ensure that the culture had reached the late-log phase. The resulting broth was serially diluted in 0.1% sterile buffered peptone water (HiMedia) to obtain a bacterial culture with a final density of 5 log CFU/mL. An inoculum of 0.1 mL of each culture was then spread-plated on the respective media. On each plate, three discs were placed equidistant from one another using sterile tweezers. An aliquot of 100 μL of the different solutions, namely, the positive control (chloramphenicol), the negative control (99% ethanol), and the extract solutions, was pipetted onto each disc. Depending on the bacterial strain used, the plate was incubated for 18–24 h at 37°C. The inhibition zones were examined after incubation using a ruler. To ensure reliability, the test was repeated three times [21].
2.3.8.2. Determination of Antimicrobial Effects Using the Minimum Inhibitory Concentration (MIC) Assay
The microdilution method was used to determine the MICs as adapted from Eloff [22]. The different bacterial strains were grown in liquid media as described above. After incubation, a loopful of the colonies was transferred to 10 mL of nutrient broth (HiMedia) and incubated for another 24 h at 37°C. After 24 h, 1 mL of the broth was transferred to 9 mL of fresh nutrient broth for incubation at 37°C for another 24 h to ensure that the culture had reached the late-log phase. The resulting broth was serially diluted in 0.1% sterile buffered peptone water (HiMedia) to obtain a bacterial culture with a final density of 5 log CFU/mL, which was used for the antibacterial screening assay [23].
Crude citrus extract suspensions of different concentrations (5, 10, 25, 50, and 100 mg/mL) were prepared for antimicrobial analysis by dissolving the extracts in distilled water. Aliquots of 100 μL of the crude citrus suspensions of different concentrations were added to the wells of the plates, followed by 100 μL of standardized inoculum (5 log CFU/mL) of the tested microorganisms. The plates were then incubated at 37°C for 24 h. An aliquot of 40 μL of 0.3 mg/mL p-iodonitrotetrazolium (INT) was then added to each well and incubated for about 30 min. The MIC was defined as the lowest concentration at which microbial growth was inhibited. A completely clear well indicated inhibition of growth, while a pink colour indicated growth in the wells. Distilled autoclaved water was used as the negative (solvent) control. For the positive control, 200 μL of the respective organisms was added to the last well of each replicate. The experiment was carried out in triplicate.
2.3.9. Data and Statistical Analysis
All experiments were performed in at least two independent replicates. LC-MS data were analyzed using MassLynx software. TPC, TFC, FRAP, CUPRAC, NO radical scavenging assay, XO inhibition, and microbial population density data were statistically analyzed using a single-factor ANOVA followed by Tukey’s post hoc test using Minitab Release 17. Statistical significance was assigned to p values less than 0.05 according to the procedure of Snedecor and Cochran [24].
3. Results and Discussion
3.1. Total Phenolic Content, Total Flavonoid Content, and Antioxidant Properties of Citrus Peel Extracts
The Rodriguan grapefruit peel showed the highest total phenolic content (64.53 ± 3.25 mg GAE/g extract) (p < 0.05) (Table 1). Elkhatim et al. [25] also found that grapefruit peels had the highest total phenolic content, followed by lemons and oranges (Table 1). Further, Li et al. [26] reported that grapefruit peels have higher total phenolic content than mandarins, lemons, and orange peels [27]. The total flavonoid content of the Rodriguan lime peel (53.48 ± 5.77 mg RE/g) and the Rodriguan grapefruit peel (49.34 ± 1.61 mg RE/g) was higher than that of the Mauritian pamplemousses (36.09 ± 0.35 mg RE/g) (p < 0.05).
| Yield % dry weight | Total phenolic content (mg gallic acid equivalents/g extract) | Total flavonoid content (mg rutin equivalents/g extract) | |
|---|---|---|---|
| Rodriguan lime | 19 | 37.17 ± 5.07b | 53.48 ± 5.77a |
| Mauritian pamplemousses | 27 | 42.80 ± 0.60b | 36.09 ± 0.35b |
| Rodriguan grapefruit | 21 | 64.53 ± 3.25a | 49.34 ± 1.61a |
- Note: Values are reported as mean ± standard deviation for three independent experiments. Different superscripts in each column represent significant difference between samples (p < 0.05).
The scavenging of radicals by antioxidants most commonly occurs via two mechanisms: the transfer of either a hydrogen atom or an electron to convert the radical into a stable compound [28]. Antioxidants neutralize radicals primarily through two key mechanisms: hydrogen atom transfer (HAT) and single-electron transfer (SET), both of which stabilize reactive species and prevent oxidative damage [28]. The FRAP assay evaluates an extract’s electron-donating capacity by measuring the reduction of Fe3+ to Fe2+ in an acidic environment, reflecting its total reducing power. Similarly, the CUPRAC assay assesses the reduction of Cu2+ to Cu+ in a near-neutral pH, making it more physiologically relevant and capable of detecting a broader range of antioxidants [29]. Both the CPURAC and FRAP assays functions through SET. The results of the FRAP and CUPRAC assays (Table 2) did not show significant differences (p > 0.05) among the three citrus extracts, suggesting comparable overall reducing power across the samples.
| Ferric reducing antioxidant power (mg TE/g extract) | Cupric ion reducing capacity (mg TE/g extract) | Nitric oxide radical scavenging (IC50mg/mL) | Xanthine oxidase inhibition (IC50 mg/mL) | |
|---|---|---|---|---|
| Rodriguan lime | 14.50 ± 3.11a | 0.71 ± 0.20a | 0.22 ± 0.01a | - |
| Mauritian pamplemousses | 12.96 ± 0.97a | 0.50 ± 0.04a | 0.18 ± 0.02b | 0.13 ± 0.00 |
| Rodriguan grapefruit | 14.77 ± 1.47a | 0.59 ± 0.11a | 0.18 ± 0.02b | - |
- Note: Values are reported as mean ± standard deviation for three experiments; “-” indicates no activity. Different superscripts in each column represent significant difference between the tested samples (p < 0.05).
- Abbreviation: TE: Trolox equivalents.
The scavenging of NO radicals by antioxidants typically involves HAT. The ability of the extracts to scavenge radicals varied, as reflected in the NO radical scavenging assay. Mauritian pamplemousses (0.18 ± 0.02) and Rodriguan grapefruit (0.18 ± 0.02) extracts exhibited significantly (p < 0.05) lower IC50 values than the Rodriguan lime (0.22 ± 0.01) in the NO radical scavenging assay. The lower NO scavenging activity of Mauritian pamplemousses and Rodriguan grapefruit compared to Rodriguan lime is likely attributed to their higher total flavonoid contents. Erba et al. [30] found a statistically significant and strong linear correlation (r = 0.975, p = 0.025) between total flavonoid content and DPPH, suggesting that antioxidant activity depends on the concentration of flavonoids present. XO primarily functions through SET [31]. Data gathered from the present study revealed that only the Mauritian pamplemousses peel extract inhibited XO activity (Table 2).
Phenolic and flavonoid compounds are produced by plants to protect themselves or promote their growth in unfavorable conditions. However, they are also reported to play an important role against oxidative stress and damage due to their radical neutralization, iron binding, and reducing capacity [32]. Phenolic and flavonoid molecules are important antioxidant components responsible for quenching free radicals based on their ability to provide hydrogen atoms [33]. They also have ideal structural characteristics for scavenging free radicals [34, 35].
3.2. In Vitro Antimicrobial Efficacy of the Citrus Peel Extracts
The disc diffusion assay was performed on a variety of spoilage organisms using the different citrus extracts. The Rodriguan grapefruit extract showed no inhibitory effect against S. aureus, Salmonella, E. coli, Listeria, Bacillus, or Penicillium (Table 3). The Mauritian pamplemousses extract showed inhibitory effects against Salmonella, E. coli, and Listeria, as shown in Table 3. Literature reports indicate that extracts from citrus peels have antimicrobial activity against a variety of microorganisms, supporting our findings [36]. The Rodriguan lime extract showed inhibitory effects against the following organisms: S. aureus (1.5 ± 0.36 cm), S. enterica (1.8 ± 0.53 cm), E. coli (2.1 ± 0.46 cm), L. monocytogenes (1.8 ± 0.7 cm), B. cereus (1.6 ± 0.69 cm), and Lactobacillus plantarum (1.3 ± 0.12 cm) (Table 3) (Figure 2). Thus, the Rodriguan lime extract showed the broadest efficacy against the tested foodborne organisms compared to the Mauritian pamplemousses and Rodriguan grapefruit extracts, which showed little to no inhibition according to the disc diffusion assay.
| Microorganisms | Solvent: dH2O (negative control) | Antibiotic: Chloramphenicol (positive control) | Rodriguan lime | Rodriguan grapefruit | Mauritian Pamplemousses |
|---|---|---|---|---|---|
| Staphylococcus aureus | 0.0 ± 0.00c | 4.6 ± 0.42a | 1.5 ± 0.36b | 0.0 ± 0.00c | 0 ± 0.00c |
| Salmonella enterica | 0.0 ± 0.00c | 4.1 ± 0.17a | 1.8 ± 0.53b | 0.0 ± 0.00c | 1.1 ± 0.1b |
| Escherichia coli | 0.0 ± 0.00c | 4.6 ± 0.58a | 2.1 ± 0.46b | 0.0 ± 0.00c | 1.4 ± 0.17b |
| Listeria monocytogenes | 0.0 ± 0.00c | 4.7 ± 0.84a | 1.8 ± 0.70b | 0.0 ± 0.00c | 0.8 ± 0.45c |
| Bacillus cereus | 0.0 ± 0.00c | 4.7 ± 0.93a | 1.6 ± 0.69b | 0.0 ± 0.00c | 0.0 ± 0.00c |
| Lactobacillus plantarum | 0.0 ± 0.00c | 4.2 ± 0.61a | 1.3 ± 0.12b | 1.4 ± 0.85c | 0.0 ± 0.00c |
| Penicillium citrinum | 0.0 ± 0.00c | 1.7 ± 0.06b | 0.0 ± 0.00c | 0.0 ± 0.00c | 0.0 ± 0.00c |
- Note: Values are reported as mean ± standard deviation for three independent trials. Different superscripts between rows represent significant difference between the tested samples (p < 0.05). Different superscripts in each column represent significant difference between the organisms tested against (p < 0.05). (+) control: chloramphenicol. (−) control: distilled H2O.
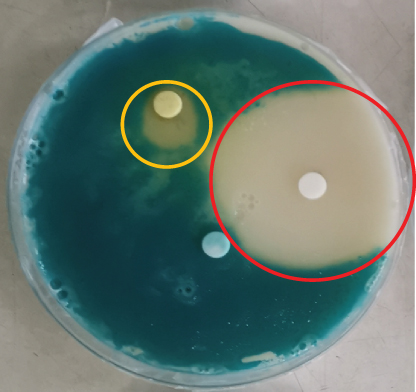
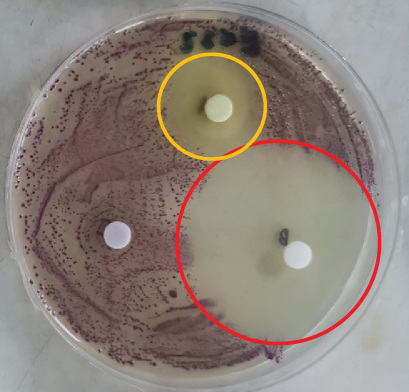
3.2.1. In Vitro Antimicrobial Assay for Different Citrus Extracts Against Several Spoilage Organisms Using Disc Diffusion Assay
The disc diffusion assay is widely known to be inaccurate since the inhibition zones need to be visually assessed with the naked eye [37–39]. On the other hand, the MIC assay is a much better technique to assess the effect of these extracts against the tested organisms. Furthermore, high concentrations (100 mg/mL) of the different extracts were needed to indicate clear inhibition zones around the inoculated discs (Figure 2), given the low sensitivity of the assay [40]. The MIC assay was subsequently used to determine more precisely the concentration of extracts effective at inhibiting microbial growth, given its greater reliability and consistency [40].
3.2.2. In Vitro Antimicrobial Assay for Different Citrus Extracts Against Several Spoilage Organisms Using the MIC Assay
Table 4 shows that the Rodriguan lime extract consistently had the lowest overall MIC, in the range of 5–10 mg/mL, against all the tested organisms except for E. coli, where the MIC was 25 mg/mL. The Rodriguan grapefruit extract and Mauritian pamplemousses extract had generally higher overall MICs of 10 and 25 mg/mL, respectively, against their tested organisms compared to the Rodriguan lime extract (5–10 mg/mL). According to Chaisiwamongkhol et al. [41], key lime extracts have been shown to inhibit a range of spoilage organisms, thereby extending the shelf life of perishable food products. They are also effective against foodborne pathogens such as E. coli, Salmonella spp., L. monocytogenes, and Staphylococcus aureus, which are responsible for serious foodborne illnesses. This dual action highlights the potential of key lime extracts as natural preservatives in the food industry, offering a safer and more sustainable alternative to synthetic chemical additives [41].
| Organisms | MIC (mg/mL) | ||
|---|---|---|---|
| Rodriguan grapefruit | Mauritian Pamplemousses | Rodriguan lime | |
| Staphylococcus aureus | 25 ± 0.0 | 25 ± 0.0 | 10 ± 0.0 |
| Salmonella enterica | 25 ± 0.0 | 10 ± 0.0 | 10 ± 0.0 |
| Escherichia coli | 25 ± 0.0 | 25 ± 0.0 | 25 ± 0.0 |
| Listeria monocytogenes | 25 ± 0.0 | 25 ± 0.0 | 10 ± 0.0 |
| Bacillus cereus | 10 ± 0.0 | 10 ± 0.0 | 5 ± 0.0 |
| Lactobacillus plantarum | 25 ± 0.0 | 25 ± 0.0 | 10 ± 0.0 |
| Penicillium citrinum | 10 ± 0.0 | 10 ± 0.0 | 5 ± 0.0 |
| Aspergillus niger | 25 ± 0.0 | 25 ± 0.0 | 10 ± 0.0 |
| Issatchenkia orientalis | 25 ± 0.0 | 25 ± 0.0 | 10 ± 0.0 |
- Note: Values are reported as mean ± STD for three independent replicates. (+) control: chloramphenicol. (−) control: distilled H2O.
3.3. Identification and Quantification of Coumarins and Flavonoids in the Citrus Peel Extracts
Table 5 shows the chromatographic and mass spectral data of coumarins and flavonoids identified in extracts from the peels of the three citrus varieties.
| tR (min) | λmax (nm) | Molecular formula (M+) | [M+] (m/z) | MS/MS (% intensity) | Proposed compound | Peak no. |
|---|---|---|---|---|---|---|
| Flavonoid derivatives | ||||||
| 2.29 | 267, 351 | C21H21O10 | 433.113 | 287.055 (100) | Kaempferol-7-O-alpha-l-rhamnoside | 1 |
| 2.76 | 282, 327 | C15H13O5 | 273.077 | 273.077 (100), 153.02 (21) | Naringenin | 2 |
| 2.83 | 267, 335 | C27H31O14 | 579.171 | 271.060 (100) | Apigenin 7-O-neohesperidoside | 3 |
| 3.02 | 285 | C28H35O15 | 611.197 | 303.089 (100), 153.018 (21) | Hesperidin | 4 |
| 3.02 | 267, 335 | C27H31O14 | 579.171 | 271.060 (100) | Apigenin 7-O-neohesperidoside isomer | 5 |
| 3.07 | 284 | C28H33O15 | 609.182 | 301.070 (100) | Diosmin (diosmetin 7-O-rutinoside) | 6 |
| 3.45 | 282, 327 | C30H35O17 | 667.187 | 273.076 (100), 463.157 (33) | 6″-O-Malonyl naringin | 9 |
| 4.04 | 332, 269 | C30H33O17 | 665.170 | 271.060 (100) | Apigenin 7-(6″-malonyl neohesperidoside) | 10 |
| 15.7 | 285 | C33H41O19 | 741.22 | 579.171 (100), 271.06 (41) | Apigenin 7-O-(2G-rhamnosyl)gentiobioside | 18 |
| 19.94 | 283 | C27H31O14 | 579.174 | 579.174 (100), 271.060 (19), 151.00 (18) | Apigenin-rutinoside | 24 |
| 21.72 | 280 | C29H33O15 | 621.182 | 271.060 (58), 151.002 (70) | Camellianin A (apigenin 7-O-glucoside 4′-acetate) | 26 |
| 22.24 | 283 | C29H33O15 | 621.183 | 621.183 (100), 151.001 (40), 271.062 (30) | Vitexin 2″-O-rhamnoside 6″-acetate | 27 |
| 24.66 | 321 | C27H31O10 | 515.192 | 515.192 (100) | Icariside II | 28 |
| Coumarin derivatives | ||||||
| 3.41 | 282, 327 | C15H17O4 | 261.113 | 189.055 (100), 243.103 (25), 131.05 (23), 103.057 (6) | Meranzin hydrate | 7 |
| 3.56 | 312 | C18H19O10 | 395.09 | 147.04 (100) | 9-Hydroxy-4-methoxypsoralen 9-glucoside | 8 |
| 4.58 | 323, 257 | C15H17O4 | 261.114 | 189.055 (100), 243.103 (25), 131.05 (23), 103.057 (6) | Auraptenol | 11 |
| 4.62 | 351 | C16H15O5 | 287.092 | 203.034 (100) | Oxypeucedanin isomer | 12 |
| 4.92 | 322 | C10H9O3 | 177.055 | 121.06 (83), 147.045 (14) | 7-Methoxycoumarin | 13 |
| 6.81 | 330 | C11H11O4 | 207.065 | 207.065 (100), 192.042 (50), 149.023 (41) | Limettin | 14 |
| 7.17 | 267, 312 | C13H11O5 | 247.060 | 217.013 (100) | Isopimpinellin | 15 |
| 7.6 | 282, 329 | C15H17O4 | 261.115 | 189.055 (100), 243.103 (25), 131.05 (23), 103.057 (6) | Meranzin | 16 |
| 7.82 | 282, 330 | C15H17O7 | 261.116 | 189.055 (100), 243.103 (25), 131.05 (23), 103.057 (6) | Isomeranzin | 17 |
| 8.10 | 322 | C21H23O5 | 355.154 | 355.154 (100), 163.039 (45), 189.055 (29), 261.111 (20) | Umbelliferone derivative | 18 |
| 8.92 | 351 | C16H15O5 | 287.092 | 203.034 (100) | Oxypeucedanin | 19 |
| 13.58 | 310 | C23H21O5 | 377.139 | 203.034 (100), 147.045 (22) | Bergamottin derivative | 20 |
| 17.75 | 271, 312 | C22H25O5 | 369.170 | 233.045 (100) | 5-Geranyloxy-8-methoxypsoralen | 21 |
| 18.03 | 309 | C11H7O4 | 339.160 | 203.034 (100) | Bergamottin | 22 |
| 18.29 | 323 | C20H25O4 | 329.175 | 329.175 (100), 193.050 (23) | 7-Geranyloxy-6-methoxycoumarin | 23 |
| 20.25 | 337, 267 | C27H29O14 | 577.154 | 269.046 (100) | Coumestrol-rutinoside | 25 |
3.3.1. Flavonoid Derivatives Identified
3.3.1.1. Kaempferol Derivatives
Peak 1, with a retention time of 2.29 min and a maximum absorption wavelength (λmax) of 267 and 351 nm, with the main fragment of m/z 433.113, was identified as kaempferol-7-O-alpha-l-rhamnoside. This identification was based on the kaempferol aglycone (m/z 287.055), corresponding to the loss of a rhamnose (−146 amu) moiety.
3.3.1.2. Apigenin Derivatives
All seven apigenin derivatives identified produced a fragment at m/z 271.090, corresponding to the apigenin molecule. Peaks 3, 5, and 24 were identified as apigenin-7-O-neohesperidoside, its isomer, and apigenin-rutinoside, respectively, based on the loss of a rutinose (−308 amu). Peak 10 was identified as apigenin 7-(6″-malonyl neohesperidoside) due to the loss of a malonyl hexose (−248 amu) and a rhamnosyl (−146 amu) moiety [−394 amu] [42]. Peak 18 was identified as apigenin 7-O-(2G-rhamnosyl) gentiobioside based on the loss of a glucose (−162 amu) unit, a rutinose (−308 amu), and the consecutive loss of a glucose (−162 amu) molecule. Peaks 26 and 27 were identified as Camellianin A (apigenin 7-O-glucoside 4′-acetate) and vitexin 2″-O-rhamnoside 6″-acetate, respectively. For both compounds, fragmentation resulted in the loss of a rhamnoside (−146 amu) and an acetyl hexose (−204 amu) fragment [42].
3.3.1.3. Naringenin Derivatives
Peak 2, with a retention time of 2.76 min, a maximum absorption wavelength (λmax) of 282 and 327 nm, and a main fragment at m/z 273.077, was tentatively identified as naringenin. Fragmentation produced fragments at m/z 153.02, corresponding to the 1,3A fragment from the retro-Diels–Alder (RDA) cleavage of the naringenin aglycone at bond Positions 1 and 3 in the C ring [43, 44]. Peak 9 was identified as 6″-O-malonyl naringin based on the loss of a malonyl hexose (−204 amu), followed by the loss of a hexose (−162 amu) moiety [42]. Peak 6, with a retention time of 3.07 min, a maximum absorption wavelength (λmax) of 284 nm, and a main fragment of m/z 609.182, was identified as diosmin (diosmetin 7-O-rutinoside). Fragmentation produced an m/z 301.070 fragment, corresponding to the loss of a rutinose (−308 amu) fragment [42]. Peak 28, with a retention time of 24.66 min and a maximum absorption wavelength (λmax) of 321 nm, was identified as Icariside II based on the main fragment of m/z 515.192.
3.3.2. Coumarin Derivatives Identified
3.3.2.1. Meranzin Derivatives
All three meranzin derivatives were identified based on the main fragment of m/z 261.113. Peaks 7, 11, 16, and 17 were identified as meranzin hydrate, auraptenol, meranzin, and isomeranzin, respectively. Peak 13, with a retention time of 4.92 min, a maximum absorption wavelength (λmax) of 322 nm, and a parent fragment of m/z 177.055, was identified as 7-methoxycoumarin based on the consecutive loss of two carbon monoxide molecules [−56 amu], producing an m/z 121.06 fragment. The m/z 147.045 fragment corresponds to the loss of a methoxy group (−30 amu) [45]. Peak 14 was identified as limettin (citropten) based on the main fragment at m/z 207.065. Further fragmentation yielded ionic fragments of m/z 192.042 and 149.023. The m/z 192.042 fragment resulted from the loss of a methyl (−15 amu) molecule, while the m/z 149.023 fragment was due to the loss of a methyl (−15 amu) followed by the loss of a C2H3O (−43 amu) moiety [−58 amu] [45].
3.3.2.2. Furanocoumarin Derivatives
Peak 19, with a retention time of 4.62 min, a maximum absorption wavelength (λmax) of 351 nm, and a parent fragment at m/z 287.092, was identified as oxypeucedanin based on the m/z 203.034, which is the hydroxy psoralen corresponding to the loss of a trimethyl epoxide (−84 amu) moiety [46]. Peak 15 was identified as isopimpinellin based on the parent fragment at m/z 247.060 and the main ionic fragment at m/z 217.013, corresponding to the consecutive loss of two methyl [−30 amu] groups [47]. Peak 18 was identified as an umbelliferone derivative based on the main fragment at m/z 163.039. Peak 20 was identified as a bergamottin derivative based on the main ionic fragment produced as a bergaptol ion at m/z 203.034 [3]. Peak 21, with a retention time of 17.75 min, a maximum absorption wavelength (λmax) of 271 and 312 nm, and a parent fragment at m/z 369.170, was identified as 5-geranyloxy-8-methoxypsoralen. Fragmentation produced ions at m/z 233.045, corresponding to the loss of a geranyl group (−136 amu) [48]. Peak 22 was identified as bergamottin based on the molecular formula C11H7O4, with a parent ion at m/z 339.160. Fragmentation produced a bergaptol ion at m/z 203.034 [3]. Peak 23 was identified as 7-geranyloxy-6-methoxycoumarin based on a main parent fragment at m/z 329.175 and the loss of a geranyl (−136 amu) moiety, resulting in an m/z 193.050 fragment [48]. Peak 25 was identified as coumestrol-rutinoside based on the coumestrol main fragment with m/z 267.046, corresponding to the loss of a rutinoside (−308 amu) moiety [42].
The Rodriguan grapefruit extract possessed the largest overall concentration of flavonoids (4821.1 mg RE/kg), followed by the Mauritian pamplemousses (3178.6 mg RE/kg) and the Rodriguan lime (1076.0 mg RE/kg) (Table 6). The major flavonoids identified in the Rodriguan grapefruit extract were apigenin-rutinoside (1481.7 mg RE/kg), naringenin (1200.1 mg RE/kg), and diosmin (diosmetin 7-O-rutinoside) (828.0 mg RE/kg). The major flavonoids identified in the Mauritian pamplemousses extract sample were apigenin-rutinoside (1221.0 mg RE/kg), naringenin (947.8 mg RE/kg), and vitexin 2″-O-rhamnoside 6″-acetate (449.0 mg RE/kg). The major flavonoids identified in the Rodriguan lime extract sample were hesperidin (661.6 mg RE/kg), Icariside II (393.81 mg RE/kg), and kaempferol-7-O-alpha-l-rhamnoside (11.54 mg RE/kg).
| Compound | Concentration (mg RE/kg) | ||
|---|---|---|---|
| Rodriguan grapefruit | Mauritian pamplemousses | Rodriguan lime | |
| Naringenin | 1200.1 | 947.8 | NI |
| Hesperidin | NI | NI | 661.6 |
| Kaempferol-7-O-alpha-l-rhamnoside | NI | NI | 11.54 |
| Apigenin 7-O-neohesperidoside | 72.05 | NI | 9.03 |
| Apigenin 7-O-neohesperidoside isomer | NI | 194.9 | NI |
| Apigenin-rutinoside | 1481.7 | 1221.0 | NI |
| 6″-O-Malonyl naringin | 385.7 | 388.9 | NI |
| Apigenin 7-(6″-malonyl neohesperidoside) | 32.6 | 39.6 | NI |
| Apigenin 7-O-(2G-rhamnosyl) gentiobioside | 30.9 | 74.4 | NI |
| Camellianin A (apigenin 7-O-glucoside 4′-acetate) | 63.8 | 54.4 | NI |
| Vitexin 2″-O-rhamnoside 6″-acetate | 497.4 | 449.0 | NI |
| Diosmin (diosmetin 7-O-rutinoside) | 828.0 | NI | NI |
| Icariside II | 228.9 | 197.4 | 393.81 |
| Total flavonoids | 4821.1 | 3178.6 | 1076.0 |
- Abbreviations: NI: not identified, RE: rutin equivalents.
From Table 6, two specific flavonoids were identified in the Rodriguan lime extract but not in the Rodriguan grapefruit or Mauritian pamplemousses samples, that is, hesperidin and kaempferol-7-O-alpha-L-rhamnoside. Hesperidin is a flavanone glycoside present in citrus peel. Akbar et al. [5] found that hesperidin exhibited notable antimicrobial, anti-inflammatory, antioxidant, and antitumor activities. Similarly, Iranshahi et al. [6] also found that hesperidin has antibacterial activity and suggested that these properties may be due to mechanisms such as the activation of the host immune system, disruption of bacterial membranes, and interference with bacterial enzymes [49]. In addition to its anticancer and anti-inflammatory effects, kaempferol and its related compounds also have antibacterial, antifungal, and antiprotozoal activities [50].
The Rodriguan grapefruit extract possessed the largest overall concentration of coumarins (13,476 mg CE/kg), followed by the Mauritian pamplemousses extract (Table 6). The major coumarins identified in the Rodriguan grapefruit extract sample were meranzin (5317.3 mg CE/kg), auraptenol (4853.8 mg CE/kg), and isomeranzin (2129.6 mg CE/kg). The major coumarins identified in the Mauritian pamplemousses sample were meranzin (3287.4 mg CE/kg), auraptenol (2900.6 mg CE/kg), and isomeranzin (827.4 mg CE/kg). The major coumarins identified in the Rodriguan lime extract sample were isopimpinellin (1731.5 mg CE/kg), 7-geranyloxy-6-methoxycoumarin (1616.5 mg CE/kg), and limettin (1372.0 mg CE/kg).
Looking at Table 7, a number of coumarins were identified in the Rodriguan lime extract but were not present in the other two peel extracts, that is, 9-hydroxy-4-methoxypsoralen 9-glucoside, 7-methoxycoumarin, limettin, isopimpinellin, 5-geranyloxy-8-methoxypsoralen, and 7-geranyloxy-6-methoxycoumarin. According to findings by Piao et al. [51], 9-hydroxy-4-methoxypsoralen displayed powerful antioxidant effects against the DPPH radical and against renal epithelial cell injury. Their results also showed that the aromatic hydroxy group plays a significant antioxidant role by stabilizing the radical form and participating in electron delocalization.
| Compound | Concentration (mg CE/kg) | ||
|---|---|---|---|
| Rodriguan grapefruit | Mauritian pamplemousses | Rodriguan lime | |
| Meranzin hydrate | 722.23 | 250.0 | NI |
| 9-Hydroxy-4-methoxypsoralen 9-glucoside | NI | NI | 35.1 |
| Auraptenol | 4853.8 | 2900.6 | NI |
| Oxypeucedanin | 159.9 | 45.5 | NI |
| 7-Methoxycoumarin | NI | NI | 127.0 |
| Limettin | NI | NI | 1372.0 |
| Isopimpinellin | NI | NI | 1731.5 |
| Meranzin | 5317.3 | 3287.4 | NI |
| Isomeranzin | 2129.6 | 827.4 | NI |
| Umbelliferone derivative | 234.7 | 162.4 | NI |
| Bergamottin derivative | 34.7 | 701.7 | NI |
| 5-Geranyloxy-8-methoxypsoralen | NI | NI | 386.5 |
| Bergamottin | NI | 46.6 | 877.7 |
| 7-Geranyloxy-6-methoxycoumarin | NI | NI | 1616.5 |
| Coumestrol-rutinoside | 183.5 | 404.0 | NI |
| Total coumarins | 13476 | 8626 | 6146 |
- Abbreviations: CE: coumarin equivalents, NI: not identified.
In fact, 7-methoxycoumarin has been recently identified by Han et al. [52], who also reported its strong antibacterial activity against Ralstonia solanacearum, a Gram-negative organism. This is congruent with our finding that Rodriguan lime extract also showed inhibitory activity against Gram-negative bacteria Salmonella and E. coli. 7-Methoxycoumarin has also been demonstrated to restrict biofilm formation, induce bacterial cell membrane lysis, and significantly suppress virulence-associated genes. Additionally, Yang et al. [53] found that 7-methoxycoumarin, which contains a methoxy functional group on the coumarin skeleton, has notable antibacterial activity against foodborne pathogens. In addition to 7-methoxycoumarin, isopimpinellin was another novel molecule identified within the Rodriguan lime. This molecule was reported to exhibit antimicrobial activity against Cryptococcus neoformans and Mycobacterium intracellulare [54]. Furthermore, Madeiro et al. [7] found that isopimpinellin potentiated the effect of erythromycin, which could be attributed to its mechanism of action as an efflux pump inhibitor of bacteria. Little information is available on the scientific application of 7-geranyloxy-6-methoxycoumarin. However, as a coumarin derivative, a type of compound found in many plants, it has been studied for its potential medicinal properties (e.g., antioxidant, anti-inflammatory, antitumor, and antimicrobial activities). Furthermore, 7-geranyloxy-6-methoxycoumarin has been found to have potential therapeutic applications in a variety of diseases, including cancer, diabetes, and Alzheimer’s [55, 56]. The antifungal activity of Rodriguan lime against Penicillium citrinum and Aspergillus niger could likely be attributed to the molecule limettin. In fact, Ramírez-Pelayo et al. [8] found that limettin exhibited inhibitory effects against the fungus Colletotrichum sp., surpassing those of known phytoalexins scoparone and umbelliferone.
Flavonoids and coumarins exhibit antimicrobial properties through diverse mechanisms, making them effective against both Gram-positive and Gram-negative bacteria. Flavonoids disrupt bacterial membranes by increasing their permeability, leading to the leakage of intracellular contents, as seen with compounds like quercetin and catechins [57]. They also inhibit essential bacterial enzymes, such as DNA gyrase and β-lactamase, which are critical for replication and survival—examples include apigenin and luteolin [58]. Additionally, flavonoids chelate vital metal ions such as iron, depriving bacteria of necessary resources for metabolic functions [59]. Furthermore, flavonoids interfere with biofilm formation, either preventing its development or disrupting existing biofilms, a key bacterial defense mechanism [57].
Since the overall concentration of coumarins and flavonoids was not the highest within the Rodriguan lime extract sample (Tables 6 and 7), its antimicrobial efficacy could likely be attributed to other specific compounds identified within the sample or the synergistic effect of coumarins and flavonoids found within the extract.
3.4. Conclusion
Rodriguan lime was found to exhibit antioxidant activity comparable to that of Mauritian pamplemousses and Rodriguan grapefruit. However, the Rodriguan lime extract exhibited a lower MIC (10 mg/mL) against several spoilage organisms compared to the other extracts tested (25 mg/mL). This could be attributed to the compounds uniquely identified within the Rodriguan lime sample. Rodriguan lime extract was found to contain several compounds not present in the Mauritian pamplemousses and Rodriguan grapefruit extracts, namely, 9-hydroxy-4-methoxypsoralen 9-glucoside, 7-methoxycoumarin, limettin, isopimpinellin, 5-geranyloxy-8-methoxypsoralen, and 7-geranyloxy-6-methoxycoumarin. Citrus peels, rich in valuable bioactive compounds like phenolics and coumarins, could serve as active ingredients in food products or even replace synthetic preservatives entirely [9]. Incorporating these bioactive compounds into an antimicrobial edible coating offers significant potential to extend shelf life, improve food safety, and minimize dependence on synthetic preservatives. Natural bioactive compounds can also be used as potential therapeutic agents that may eventually replace antibiotics that are no longer effective against bacterial pathogens.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ms. Liza Cloete: conceptualization, methodology, visualization, writing–original draft, writing–review and editing, formal analysis. Prof. Kwaku Gyebi Duodu: writing–review and editing, validation. Prof. Mohammad Naushad Emmambux: conceptualization, supervision, writing–review and editing, validation. Dr. Anton Venter: methodology, writing–original draft, writing–review and editing. Dr. Deena Ramful-Baboolall: conceptualization, supervision, writing–review and editing, validation. Associate Prof. Dr. Brinda Ramasawmy: conceptualization, supervision. Dr. Swaleha Hudaa Neetoo: writing–review and editing, validation. Dr. Carene Picot-Allain: methodology, writing–review and editing.
Funding
This work was funded by the Higher Education Commission (HEC) (project vote T0712.F01294) of Mauritius and DSI (Department of Science and Innovation)-NRF (National Research Foundation) Centre of Excellence in Food Security SMART food project grant number 91490 in South Africa. The Ministry of Education, Tertiary Education, Science and Technology in Mauritius is acknowledged for providing a bursary for the first author under the Mauritius-Africa scholarship (Reference number: MTE/CR/35/12T).
Open Research
Data Availability Statement
Data is available on request from the authors.




