Characterization of the physicochemical properties of mango (Mangifera indica L., Dragon variety) kernel fat extracted via supercritical carbon dioxide and Soxhlet techniques
Abstract
Mango kernel fat (MKF) that is high in monounsaturated triacylglycerols is a potential cocoa butter alternative. This study aimed to investigate the yield and physicochemical properties of Dragon variety (Mangifera indica L.) MKF is extracted by supercritical carbon dioxide and Soxhlet. The fatty acid constituent, triacylglycerol constituent, melting and crystallization behavior, and solid fat content (SFC) were analyzed using gas chromatography, high-pressure liquid chromatography, and differential scanning calorimetry, respectively. The yield was 6.59%–9.65% depending on temperature, pressure, and time. Physicochemical properties for both techniques were comparable. The MKF contained higher levels of stearic acid (primarily StOSt, 1,3-distearoyl-2-oleoyl-glycerol) with extraction conditions of 72°C, 30 MPa, and 3 h. Melting and crystallization peaks ranged from 9.84 to 39.13°C and 16.69 to −11.23°C, respectively. SFC was zero between 40 and 45°C. The high StOSt content can aid in fractionation and demonstrates potential in making heat-resistant fat for use in confectioneries.
Novelty impact statement
The current study investigated the effect of different extraction techniques and conditions on the percentages of fat yield of the Dragon variety mango kernel (Mangifera indica L.), a by-product of the fruit industry, which has not been previously studied. The highest percentage of fat yield was 9.65% ± 1.1 at 60°C, 30 MPa and 4 h where the yield was comparable and yet was achieved at more energy-efficient extraction conditions than in previous studies. The current study provides a better understanding of the physicochemical properties of Dragon variety mango kernel fat for various food applications.
1 INTRODUCTION
Mango (Mangifera indica L.) is one of the most popular tropical fruits. According to the Food and Agriculture Organization, mango represents 52% of global tropical fruit trade (Altendorf, 2019). Among Asian countries, India is one of the top mango producers, contributing 38% of global mango production. In Malaysia, mango production has increased up to 30% during 2012–2017 (Altendorf, 2019).
After using the fruit flesh for jam, jelly, or juices, mango seeds are generally disposed of as waste (Jahurul, et al., 2014b). Depending on the variety, the mango kernel comprises 10%–25% of the whole fruit's weight and 45%–75% of the seed (Abdalla et al., 2007a). Because of its distinct fatty acid (FA) and triacylglycerol (TAG) composition, mango kernel fat (MKF) can be used as a cocoa butter alternative (CBA), and can also be used in margarine, trans-free shortening, and value-added food products (Jin et al., 2019). MKF is also reported to have high micronutrient and antioxidant properties (Abdalla et al., 2007b).
As different mango varieties vary widely in FA and TAG content, their usage varies according to the variety's physicochemical properties. In previous studies, some mango varieties from China, India, Kenya, Thailand, Malaysia, and Tanzania were examined (Abdalla et al., 2007a; Jeyarani et al., 2015; Jin et al., 2019; Muchiri et al., 2012; Sonwai & Ponprachanuvut, 2014; Tran et al., 2015). MKF content varies from 5.73% to 11.14%, depending on mango variety, maturity, and weather conditions (Jahurul, et al., 2014b; Jin et al., 2016; Sonwai & Ponprachanuvut, 2014). Palmitic, stearic, and oleic acids are three prominent FAs which vary in the range of 1.33%–11.2%, 31.3%–58.08%, and 17.99%–55.4%, respectively (Jeyarani et al., 2015; Wu et al., 2015; Yadav et al., 2017). For TAG profiles, SUS (S = saturated, U = unsaturated) content varies from 43.1% to 78% depending on country of origin. Studies have highlighted that MKF which contains more than 65% StOSt (St = stearic, O = oleic) can be used as a cocoa butter equivalent (CBE), whereas MKF below 50% has the potential to be used for fractionation, margarines, or shortenings (Gunstone, 2011; Reddy & Jeyarani, 2001; Solis-Fuentes & Duran-de-Bazua, 2004).
Soxhlet is one of the popular methods for oil extraction from fruit kernels. However, this technique uses large solvent quantities, and some solvents are hazardous to health (Abdalla et al., 2007a; Jahurul, et al., 2014b). An alternative extraction technique is supercritical carbon dioxide (SC-CO2) extraction which is growing in popularity as a green technology (Abbas et al., 2008; Danlami et al., 2014). Different extraction techniques can have an influence on extraction yield as well as the FA profile. In the case of SC-CO2, different extraction conditions such as temperature and pressure can affect the solubility of FA and TAG (Azmir et al., 2014; Zaidul et al., 2007). The optimum extraction conditions for maximizing the yield of MKF have been reported previously (Akanda et al., 2015).
Kernels are by-products of the fruit industry. The Dragon variety mango is three to four times bigger than the Waterlily variety mango and contains a larger kernel. The fat content could probably be higher in a larger kernel. The potential use of the kernel fat in the food and cosmetics industry could contribute to waste reduction. The usage of MKF varies depending on its yield, FA, and TAG profile which are affected by the extraction conditions. To date, the physicochemical properties of the kernel of Dragon mango have yet to be examined. In addition, the effect of extraction conditions on the properties of MKF yield is unclear. This study aims to investigate and compare the effect of different time, temperature, and pressure conditions of SC-CO2 technology on the yield and physicochemical properties of MKF extracted from mangoes of the Dragon variety.
2 MATERIALS AND METHODS
2.1 Materials and chemicals
Dragon variety mangoes were collected from the local market of Sekinchan, Selangor, Malaysia. Ethanol (99.6%, Fisher Scientific), n-hexane (99%, Fisher Scientific), and anhydrous methanol (99.8%, Fisher Scientific) were purchased. Instrumental grade CO2 was used (min purity 99%) from Alpha Gas Solution Sdn Bhd. FA standards for gas chromatography (GC) analysis and triacylglycerol standards for high-performance liquid chromatography (HPLC) analysis were purchased from Sigma-Aldrich, Germany. All solvents and chemicals used were of HPLC or analytical grade (Fisher Scientific). To compare the properties of MKF with cocoa butter (CB), commercial CB was purchased from Le Bourne Sdn Bhd, Malaysia.
2.2 Preparation of mango kernel powder for experiment
Mango kernels were separated manually, washed with water, and dried in the oven at 50°C until a consistent weight was achieved. Grinding was then performed using Panasonic grinder (model: MX-AC 2105) and sieving with a 200 μm sieve to achieve uniform particle size. The kernels were kept in a desiccator for a maximum of 1 day until further analysis.
2.3 Soxhlet extraction method
A sample (15 g) of mango kernel powder and 250 ml of n-hexane were used to perform the extraction for 8 h. A rotary vacuum evaporator was used to evaporate the solvent from the extracted fat. The extract was dried in an oven at 60°C for an hour and it was kept in a refrigerator for a maximum of one to 2 days until further analysis.
2.4 SC-CO2 extraction method
The supercritical fluid extractor is comprised of a CO2 cylinder, SC-CO2 extractor, a high-pressure-syringe pump with a maximum capacity of 32.5 MPa, and a chiller. A sample (4–5 g) of ground mango kernel was placed in a vessel and soaking was carried out at different temperatures (60 and 72°C), time (3, 4 and 5 h), and pressure (25 and 30 MPa) conditions. These parameters were selected and modified from Jahurul, et al., (2014b). Ethanol (95%) was used as a modifier. Previously, the addition of a polar co-solvent with CO2 flow showed significant improvement in oil extraction through SC-CO2 extraction; and ethanol was reported as the better option among other polar co-solvents based on yield and fatty acid composition (Asep et al., 2013). A rotary evaporator was then used to evaporate the extracted ethanol from the extract followed by subsequent air-drying in the fume hood to remove trace amounts of ethanol. The yield was calculated using the following equation:
2.5 Sample identities in different conditions
Table 1 presents sample identities at various extraction temperatures, pressures, and time durations using SC-CO2 alongside a sample that was extracted via the Soxhlet extraction method.
| Sample name | Pressure (MPa) | Temperature (°C) | CO2 flow (ml/min) | Time (h) | Co-solvent flow (ethanol—ml/min) |
|---|---|---|---|---|---|
| S101 | 25 | 60 | 4 | 3 | — |
| S1 | 25 | 60 | 4 | 4 | 0.5 |
| S2 | 25 | 72 | 4 | 4 | 0.5 |
| S3 | 30 | 60 | 4 | 4 | 0.5 |
| S4 | 30 | 72 | 4 | 4 | 0.5 |
| S5 | 25 | 60 | 4 | 5 | 0.5 |
| S6 | 25 | 72 | 4 | 5 | 0.5 |
| S7 | 30 | 60 | 4 | 5 | 0.5 |
| S8 | 30 | 72 | 4 | 5 | 0.5 |
| S9 | 25 | 60 | 4 | 3 | 0.5 |
| S10 | 25 | 72 | 4 | 3 | 0.5 |
| S11 | 30 | 60 | 4 | 3 | 0.5 |
| S12 | 30 | 72 | 4 | 3 | 0.5 |
| Soxhlet | 8 h (with n-hexane) | ||||
2.6 Fatty acid methyl ester analysis
The FA composition of the extracted fats was analyzed by GC with a flame ionized detector using the fatty acid methyl ester (FAME) technique. CB was used as a control sample for comparison.
Approximately, 50 mg of sample was placed in a 2 ml vial, and 0.95 ml of n-hexane was added and mixed to dissolve the fat. 0.05 ml of 1 M sodium methoxide in anhydrous methanol was added to the vial and shaken vigorously for 6–7 s in a vortex mixer. After sodium glycerolate was sedimented, 1 μl of fatty acid methyl ester was taken from the clear supernatant. It was injected into a gas chromatograph (PerkinElmer Clarus 500 GC) equipped with an elite FFAP column (30 m length × 0.32 mm internal diameter × 0.25 μm film thickness). The temperatures for injection, detection, and oven for the sample were selected by following Biswas et al., (2016). For identification of the peaks, retention times were compared to the FAME standards. Quantification was conducted using peak area normalization.
2.7 Triacylglycerol analysis
HPLC was used to determine the triacylglycerol (TAG) composition. An Agilent HPLC series 1260, CA, USA equipped with Column ZORBAX C-18 (4.6 × 250 mm, 5 mm, Agilent Technologies) was used and column oven temperature was maintained at 35°C for the analyses. Methods (Ce 5b-89) followed established procedures developed by the American Oil Chemists' Society. Acetone and acetonitrile were used as the mobile phase at a 70:30 (v/v) ratio and isocratic elution was set at a 1.0 ml/min flow rate. The sample injection volume was at 10 μl of 5% (w/v) oil in acetone. TAG peaks were identified according to the retention time of the standards, and the peak area of the chromatograms was used to calculate the peak area of the TAGs.
2.8 Differential scanning calorimetry analysis
Fat melting behavior was analyzed using differential scanning calorimetry (DSC; Pyris 4000 DSC; PerkinElmer). Approximately, 5–10 g of each sample was hermetically sealed in an aluminum pan and an empty covered pan was used as a reference. Nitrogen gas was used at a flow rate of 20 ml/min. The temperature scan followed methods previously reported by Biswas et al., (2016).
2.9 Solid fat content analysis
Solid fat content (SFC) was analyzed using peak area percentage calculation of the melting curve in Pyris software with DSC. Calculation of solid fat was performed by subtracting liquid fat content from 100 for each temperature.
2.10 Statistical analysis
Except the SC-CO2 extraction, all analyses were performed in triplicate and the mean value and standard deviation are reported. Statistical Package for Social Sciences (SPSS) software v23.0 was used for statistical analysis. The percentage of FA and TAG was compared using analysis of variance (ANOVA) with a 5% level of significance. Tukey tests were performed to compare the mean variations.
3 RESULTS AND DISCUSSION
3.1 Extraction yield
Fat extraction was performed using SC-CO2 extraction and Soxhlet extraction with n-hexane as the solvent. SC-CO2 extraction was conducted for 3, 4, and 5 h with two different temperatures and pressures (60 or 72°C; 25 or 30 MPa, respectively). In all the conditions, CO2 flow was 4 ml/min and co-solvent flow was 0.5 ml/min (Table 1).
Without co-solvent, no yield was observed in this experiment. The solvent capacity of SC-CO2 is modifiable by changing temperature and pressure. Another way to improve solvent capacity is by adding co-solvents with higher polarity which may help to enhance the bond separation efficiency, producing higher extraction of yield by SC-CO2 (Brondz et al., 2017; Lee et al., 2012; Nguyen et al., 2011). As such, ethanol was used as a co-solvent in the subsequent experiments, as it was reported to be the most efficient solvent for SC-CO2 extraction for CB extraction (Asep et al., 2013).
Figure 1 shows the yields obtained under different extraction conditions. The final yield percentages for SC-CO2 (S1–S12) and Soxhlet extraction of MKF varied between 6.59% and 9.65% (Figure 1). The yield was not significantly different under different extraction conditions. The highest yield was recorded for S3 (60°C, 4 h and 30 MPa) and lowest for S9 (60°C, 3 h and 25 MPa). These yields are comparable to previous studies on different mango varieties from Malaysia, Thailand, and China (Jahurul, et al., 2014b; Jin et al., 2016). The Dragon variety consisted higher fat content compared to the previous reports for three Chinese mango varieties (5.65%–6.75%), three Thai varieties (5.73%–6.40%), and 13 Indian mango varieties (3.70%–6.50%; Jin et al., 2016; Lakshminarayana et al., 1983; Sonwai & Ponprachanuvut, 2014). In contrast, some mango varieties were reported to have higher fat contents. These findings were for 2 Costa Rican varieties (10.70%–11.90%), 11 Indian varieties (9.90%–12.60%), and 3 Kenyan varieties (9.70%–10.40%; Lakshminarayana et al., 1983; Lieb et al., 2019; Muchiri et al., 2012). Besides geographical variations, the maturity of the fruit also affects the fat content where ripe mangoes have higher fat content than unripe mangoes (Jahurul et al., 2020).
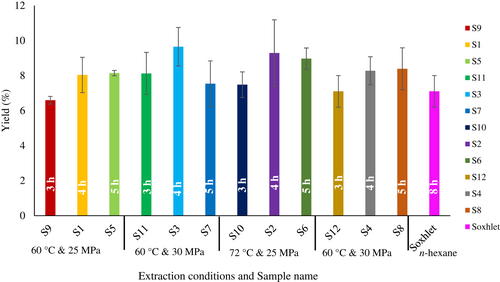
In this study, the rapid extraction phase was up to 4 h. After that, the yield percentage declined or remained equal for each extraction condition. This decline could be due to the coagulation of waxy materials in the exit of the automatic back pressure regulator (ABPR). Similar observations were reported by Machmudah et al., (2008) and Yin et al., (2005).
At 60°C, when pressure increased from 25 to 30 MPa, the yield percentage increased. However, when the temperature increased to 72°C, the yield percentage decreased. Possible reasons could include the increasing volume of CO2 with temperature, which leads to a reduction of its density. As a result, the solute loses the opportunity to dissolve (Machmudah et al., 2008; Yin et al., 2005). However, the 5 h extraction yield might be reduced due to waxy materials coagulation at ABPR from the longer time period (Sako et al., 1994). Therefore, 60°C and 30 MPa pressure for 4 h provided the best conditions for achieving the highest yield extraction.
Soxhlet and SC-CO2 extraction yields were comparable and in agreement with previous studies (Jeyarani et al., 2015; Jin et al., 2016). However, Soxhlet extraction consumed five times more solvent (n-hexane) and required more time (8 h) compared to SC-CO2 extraction.
3.2 Fatty acid composition
As the yield percentages did not vary significantly among the conditions (Figure 1), 3 and 5 h of extraction time were selected in the subsequent studies. Table 2 represents the FAs of extracted fats by the SC-CO2 and Soxhlet methods. CB was used as a control. Palmitic acid (C16), stearic acid (C18), oleic acid (C18:1), and linoleic acid (C18:2) are the four predominant fatty acids of MKF. Among them, C18 and C18:1 are the major fatty acids of MKF.
| Sample | Palmitic acid (C16) | Stearic acid (C18) | Oleic acid (C18:1) | Linoleic acid (C18:2) | Linolenic acid (C18:3) | Others |
|---|---|---|---|---|---|---|
| S5 | 7.03 ± 0.13b | 47.86 ± 0.02bc | 39.23 ± 0.19b | 5.31 ± 0.08ab | 0.37 ± 0.02 | 0.49 |
| S6 | 7.08 ± 0.06b | 47.29 ± 0.09bc | 39.54 ± 0.05b | 5.59 ± 0.02a | 0.42 ± 0.01 | 0.08 |
| S7 | 7.34 ± 0.15b | 46.06 ± 0.27c | 41.43 ± 0.11b | 5.17 ± 0.31ab | — | — |
| S8 | 6.95 ± 0.17b | 48.8 ± 0.49ab | 39.87 ± 0.90b | 4.41 ± 0.71b | 0.41 ± 0.01 | 0.11 |
| S9 | 6.89 ± 0.14b | 47.47 ± 0.15bc | 39.74 ± 0.70b | 5.53 ± 0.13ab | 0.43 ± 0.01 | 0.16 |
| S10 | 7.26 ± 0.54b | 48.26 ± 0.11abc | 39.96 ± 1.17b | 5.53 ± 0.40ab | 0.42 ± 0.03 | 0.12 |
| S11 | 6.32 ± 0.57b | 48.41 ± 0.86abc | 40.25 ± 0.71b | 4.76 ± 0.71ab | 0.4 ± 0.01 | 0.10 |
| S12 | 5.64 ± 0.01b | 50.53 ± 0.47a | 38.56 ± 0.29b | 4.71 ± 0.19ab | 0.38 ± 0.01 | 0.49 |
| Soxhlet | 7.57 ± 0.99b | 48.04 ± 0.14abc | 38.59 ± 0.32b | 5.31 ± 0.56ab | 0.38 ± 0.06 | 0.17 |
| CB | 27.7 ± 0.93a | 36.39 ± 0.65d | 33.13 ± 0.03a | 2.73 ± 0.28c | — | — |
- Note: Each value represents mean ± standard deviation of three replicates. Different letters as superscripts in the same column represent significant statistical differences (p < 0.05).
The percentage of FAs extracted using Soxhlet extraction was comparable with that of SC-CO2 extracted FAs. All the extracts contained stearic and oleic acid concentrations ranging from 46.06% to 50.53% and 38.56% to 41.43%, respectively. Palmitic and linoleic acid percentages varied between 5.64% to 7.57% and 4.41% to 5.59%, respectively (Table 2).
At different extraction conditions, palmitic and oleic acid percentages did not vary significantly among the samples. The percentages of stearic acid in S8 and S12 samples increased (p < 0.05) with increasing temperature and pressure. However, when pressure was increased from 25 to 30 MPa at 72°C, the percentage of linoleic acid decreased for 5 h extractions.
The solubility of FAs decreases with increasing carbon chain length as well as with increasing molecular double bonds (Azmir et al., 2014). Solubility also varies with CO2 density which is influenced by temperature and pressure. Previously, it was reported that stearic acid solubility increases with the increased temperature and pressure which is in agreement with this study's findings (Maheshwari et al., 1992).
The percentages of palmitic acid, stearic acid, and oleic acid in this study were also comparable with previous studies (Abdalla et al., 2007a; Jahurul et al., 2015; Jahurul, et al., 2014b; Sonwai et al., 2014; Sonwai & Ponprachanuvut, 2014; Wu et al., 2015). The palmitic acid content was in line with the Malaysian Apple, Waterlily, and Arumanis mango varieties and four Thai varieties (Jahurul, et al., 2014b; Sonwai & Ponprachanuvut, 2014). Stearic acid content was comparable to Thai mango, the Maha Chanook, and the Nam Dok Mai varieties (Lieb et al., 2019). The Dragon variety mango contains higher percentages of stearic acid (46.06%–50.53%) compared to Latin American varieties (35.10%–38.50%) and 36 Indian varieties (27%–45%) (Lakshminarayana et al., 1983; Lieb et al., 2019). Oleic acid content (38.56%–41.43%) of the Dragon mango kernel was comparable to the Malaysian Manila variety (38.14%), Thai Nam Dok Mai, Keaw-Sawoey, and Falan varieties (38.80%–41.60%), and six Indian varieties (38%–41%; Jahurul et al., 2020; Lakshminarayana et al., 1983; Lieb et al., 2019; Sonwai & Ponprachanuvut, 2014). In contrast, the three Thai varieties and four Kenyan varieties had a higher content of oleic acid compared to the Dragon variety mangoes in this study (Muchiri et al., 2012; Sonwai & Ponprachanuvut, 2014). The sum of percentage of palmitic acid, stearic acid, and oleic acid is 93%–95%, which is also comparable to previous literature, although the individual percentages varied slightly (Abdalla et al., 2007a; Jahurul, et al., 2014b). The palmitic acid content was lower than CB, palm oil, and illipe butter; higher compared to kokum butter; and within the range of shea butter (Gunstone et al., 1994; Minifie, 2012; Rui, 2012). Compared to other fats, CB also constituted 93%–95% of C16, C18, and C18:1 FAs in total. However, the individual percentage of FA for MKF differed from CB.
3.3 TAG composition
TAGs are of particular interest as they are related to melting and crystallization profiles. Three major TAGs were identified in the Dragon variety of MKF. These were 1,3-dipalmitoyl-2-oleoylglycerol (POP), 1-palmitoyl-2-oleoyl-3-stearoyl-glycerol (POSt), and 1,3-distearoyl-2-oleoyl-glycerol (StOSt). Table 3 shows the percentages of these three main TAGs from SC-CO2 and Soxhlet extractions. No significant difference was observed for POP, POSt, and StOSt between SC-CO2 and Soxhlet extracted fats.
| Sample | POP (%) | POSt (%) | StOSt (%) |
|---|---|---|---|
| S5 | 9.90 ± 0.28bc | 30.96 ± 1.34b | 42.42 ± 2.13a |
| S6 | 10.14 ± 1.04bc | 30.55 ± 2.06b | 40.70 ± 0.90bc |
| S7 | 10.53 ± 0.64b | 31.61 ± 0.75b | 39.69 ± 0.37c |
| S8 | 10.50 ± 0.42bc | 30.45 ± 0.38b | 43.53 ± 1.11a |
| S9 | 9.71 ± 0.40bc | 31.56 ± 0.45b | 41.70 ± 0.57abc |
| S10 | 9.90 ± 0.03bc | 32.27 ± 0.23b | 40.87 ± 0.89bc |
| S11 | 9.18 ± 0.44bc | 32.78 ± 0.11b | 41.73 ± 0.16abc |
| S12 | 8.93 ± 0.57c | 31.34 ± 0.38b | 44.23 ± 0.11a |
| Soxhlet | 9.41 ± 0.20bc | 30.99 ± 0.36b | 42.54 ± 0.28a |
| CB | 19.97 ± 0.25a | 39.85 ± 0.57a | 26.59 ± 0.22d |
- Note: Each value represents mean ± standard deviation of three replicates. Different letters as superscripts in the same column represent significant statistical differences (p < 0.05).
Percentages of POP and POSt obtained under different extraction conditions did not vary significantly (Table 3). However, they varied significantly from CB. StOSt percentages increased (p < 0.05) at 72°C and 30 MPa for 3 and 5 h extractions. A similar increment was also observed for stearic acid (Table 2). At higher temperature (72°C) and pressure (30 MPa), the higher molecular weight fatty acids were more soluble compared to a lower temperature and pressure extractions. S8 and S12 contained higher percentages of stearic acid, which may have contributed to the higher StOSt content in these samples. StOSt percentages vary from 28.9% to 55.4% in different mango varieties of Malaysia and China (Jahurul et al., 2015; Jin et al., 2016; Sonwai et al., 2014). The Dragon variety contained lower percentages of StOSt (39.69%–44.23%) compared to Vietnamese mango fat (66.3%); however, it is comparable to Indian mango fat (38.1%; Tran et al., 2015). The high StOSt percentage (Table 3) in the Dragon variety reflects the high steric acid content (Table 2). In both cases, the temperature and pressure conditions were the highest at 72°C and 30 MPa for this study. The Dragon variety contained higher percentages of POP (8.93%–10.53%) compared to a previous study on Thai mango variety by Sonwai et al., (2014). Comparing the percentages of POP, POSt, and StOSt of MKF with CB, the percentage of StOSt was higher for all extraction conditions for MKF. However, the overall percentage of TAGs was higher for CB at all the extraction conditions compared to MKF.
3.4 Melting and crystallization profile
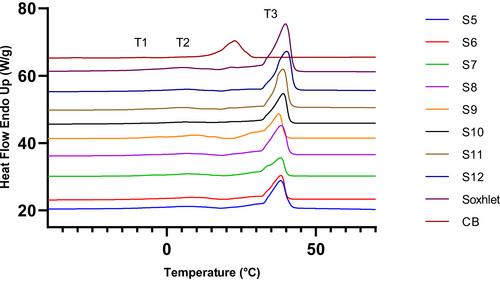
Melting and crystallization are two important physical properties of fats and oils which are influenced by the FA and TAG profile of fats. The DSC melting profile is presented in Figure 2 and Table 4. The major melting peaks for all the extracts were found in the temperature range 33.78–36.05°C. The onset temperature varied between 28.61 and 31.49°C and ended by 36.17 to 39.13°C. Before the major melting peaks, all samples exhibited small melting peaks around 5.18–6.55°C. These peaks ended between 10.08 and 14.14°C. CB melting peak and end temperatures were 32.30 and 35.42°C. The major melting peak appeared at a higher temperature compared to the melting peak temperature for CB. In the case of Soxhlet extraction, all onset, peak, and offset temperatures were comparable to SC-CO2 extracted fats. These initial onset and final offset temperatures were comparable to those of other MKFs from Malaysian and Mexican mango varieties (Jahurul et al., 2015; Solis-Fuentes & Duran-de-Bazua, 2004). However, the offset temperature was higher compared to the Chinese varieties as reported by Jin et al., (2017). The melting enthalpy (76.27–90.53 J/g) was higher compared to previous literatures reported for MKF, which indicated a higher temperature was needed to melt the crystals. This could be related to the higher percentage of StOSt presence in the Dragon variety (Table 3). Usually, the melting of crystals with higher stabilities requires more energy (Tan & Che Man, 2002). Different polymorphic forms melt over a range of temperatures, where α crystals melt at 23.5°C whereas β' and β crystals melt at 33 to 34°C (Loisel et al., 1998). Therefore, a high melting temperature of the Dragon variety mango could possibly be related to the presence of more stable crystals.
| Sample | Onset (T1) (°C) | Peak (P1) (°C) | Offset (O1) (°C) | Onset (T2) (°C) | Peak (P2) (°C) | Offset (O2) (°C) | Onset (T3) (°C) | Peak (P3) (°C) | Offset (O3) (°C) | Enthalpy (J/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| S5 | −11.12 ± 0.37 | −7.59 ± 0.18 | −4.76 ± 0.66 | −0.84 ± 0.41 | 5.87 ± 0.15 | 13.41 ± 3.82 | 29.84 ± 1.64 | 34.39 ± 2.02 | 36.55 ± 2.44 | 76.70 ± 7.33 |
| S6 | −10.28 ± 0.36 | −6.94 ± 2.14 | −3.69 ± 1.46 | −1.42 ± 1.69 | 6.44 ± 2.30 | 14.14 ± 0.69 | 30.78 ± 0.11 | 35.99 ± 0.73 | 38.12 ± 0.49 | 78.51 ± 11.72 |
| S7 | −10.91 ± 1.07 | −7.42 ± 0.56 | −4.62 ± 0.77 | −1.24 ± 0.45 | 5.47 ± 0.59 | 14.11 ± 0.09 | 29.79 ± 1.60 | 35.45 ± 0.51 | 37.85 ± 0.49 | 87.30 ± 2.91 |
| S8 | −10.60 ± 0.85 | −7.14 ± 0.63 | −4.41 ± 0.48 | 1.23 ± 2.71 | 6.13 ± 0.62 | 11.64 ± 2.51 | 29.74 ± 0.64 | 33.78 ± 1.88 | 36.17 ± 2.77 | 76.27 ± 7.96 |
| S9 | −10.53 ± 0.56 | −6.38 ± 1.22 | −3.76 ± 1.56 | 0.04 ± 1.36 | 6.55 ± 1.19 | 13.29 ± 4.36 | 31.49 ± 2.96 | 35.99 ± 0.77 | 38.39 ± 0.93 | 84.62 ± 11.72 |
| S10 | −10.85 ± 0.47 | −8.52 ± 0.29 | −6.48 ± 0.04 | −1.65 ± 0.45 | 6.13 ± 0.76 | 12.02 ± 0.80 | 29.95 ± 0.86 | 36.05 ± 0.94 | 39.06 ± 0.91 | 82.04 ± 4.69 |
| S11 | −9.84 ± 0.35 | −6.33 ± 0.85 | −3.70 ± 1.26 | 1.44 ± 0.20 | 6.32 ± 0.23 | 12.02 ± 3.16 | 33.01 ± 0.76 | 36.49 ± 0.04 | 39.13 ± 0.09 | 82.04 ± 1.69 |
| S12 | −10.91 ± 0.25 | −7.49 ± 0.49 | −4.33 ± 0.02 | −0.63 ± 1.06 | 6.36 ± 0.41 | 10.46 ± 0.62 | 28.61 ± 0.48 | 35.66 ± 1.89 | 39.04 ± 0.84 | 90.53 ± 4.67 |
| Soxhlet | −11.29 ± 0.30 | −7.77 ± 0.70 | −4.43 ± 1.17 | −0.22 ± 1.41 | 6.05 ± 0.93 | 10.08 ± 0.26 | 30.21 ± 1.83 | 35.19 ± 2.72 | 37.19 ± 2.80 | 80.21 ± 1.84 |
| CB | 13.26 ± 0.1 | 20.65 ± 0.30 | 24.31 ± 0.59 | 30.21 ± 1.63 | 32.30 ± 1.82 | 35.42 ± 1.22 | 58.93 ± 3.91 |
- Note: Each value represents mean ± standard deviation of three replicates.
The presence of high and low melting TAGs may be responsible for the broad range of melting profiles for MKF. Soo et al., (2020) stated that the melting temperatures of saturated TAGs are higher than the unsaturated ones. Therefore, the higher melting temperatures and melting enthalpies for MKF can be associated with the StOSt percentages as all extracts showed higher StOSt percentages compared to CB (Table 3). These samples may also have contained some trisaturates since high stearic acid percentages were observed in MKF samples (Table 2). Comparing the peak temperatures, the first peak corresponds to the low melting point of polyunsaturated fatty acids, the second peak represents the monounsaturated fatty acids, and the third peak indicates the presence of mixed triacylglycerides with different melting points (Nzikou et al., 2010).
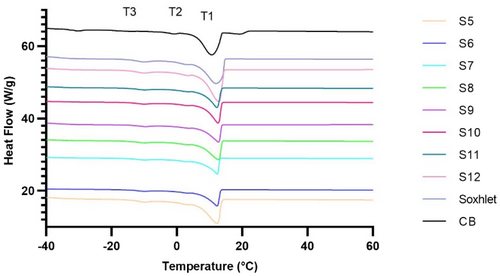
Crystallization conditions influence crystal size, shape, and numbers, which in turn influence the final product properties (Zhang et al., 2013). Crystallization thermograms and transition points of the extracted MKFs are shown in Figure 3 and Table 5. Like the melting thermograms, crystallization thermograms showed one major peak (T1) with some small peaks (Figure 3) which indicated the presence of heterogeneity of the TAGs in MKF as different TAGs can be present in various polymorphic forms.
| Sample | Onset (T1) (°C) | Peak (P1) (°C) | Offset (O1) (°C) | Onset (T2) (°C) | Peak (P2) (°C) | Offset (O2) (°C) | Onset (T3) (°C) | Peak (P3) (°C) | Offset (O3) (°C) | Enthalpy (J/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| S5 | 16.09 ± 0.29 | 14.77 ± 0.3 | 9.78 ± 0.03 | 6.73 ± 0.05 | 5.36 ± 0.07 | 3.19 ± 0.35 | −4.92 ± 0.18 | −7.81 ± 0.08 | −11.25 ± 0.20 | 35.33 ± 3.43 |
| S6 | 15.55 ± 0.08 | 14.44 ± 0.13 | 10.01 ± 0.09 | 6.77 ± 0.08 | 5.22 ± 0.10 | 2.83 ± 0.3 | −4.96 ± 0.09 | −7.91 ± 0.02 | −11.32 ± 0.03 | 33.27 ± 1.14 |
| S7 | 15.52 ± 0.26 | 14.71 ± 0.19 | 10.40 ± 0.5 | 6.67 ± 0.19 | 5.13 ± 0.25 | 2.93 ± 0.39 | −4.84 ± 0.33 | −7.77 ± 0.16 | −11.23 ± 0.18 | 30.59 ± 0.62 |
| S8 | 16.08 ± 0.08 | 14.93 ± 0.18 | 9.59 ± 0.36 | 6.75 ± 0.12 | 5.28 ± 0.18 | 2.88 ± 0.31 | −4.97 ± 0.06 | −7.84 ± 0.18 | −11.37 ± 0.08 | 40.74 ± 2.78 |
| S9 | 15.79 ± 0.26 | 14.88 ± 0.21 | 9.57 ± 0.40 | 6.89 ± 0.19 | 5.45 ± 0.16 | 3.20 ± 0.06 | −4.78 ± 0.12 | −7.75 ± 0.13 | −11.33 ± 0.17 | 39.13 ± 2.47 |
| S10 | 15.98 ± 0.16 | 14.92 ± 0.06 | 10.21 ± 0.51 | 6.62 ± 0.03 | 5.27 ± 0.07 | 2.93 ± 0.15 | −4.65 ± 0.14 | −7.93 ± 0.05 | −11.56 ± 0.07 | 40.01 ± 1.95 |
| S11 | 15.69 ± 0.11 | 14.67 ± 0.17 | 9.67 ± 0.51 | 6.51 ± 0.33 | 5.39 ± 0.13 | 3.14 ± 0.06 | −4.83 ± 0.10 | −7.85 ± 0.13 | −11.34 ± 0.13 | 34.55 ± 2.73 |
| S12 | 16.23 ± 0.81 | 15.23 ± 0.17 | 9.97 ± 0.13 | 6.99 ± 0.22 | 5.39 ± 0.14 | 2.94 ± 0.15 | −4.87 ± 0.14 | −7.90 ± 0.15 | −11.45 ± 0.21 | 40.75 ± 0.26 |
| Soxhlet | 16.69 ± 0.23 | 14.53 ± 0.04 | 8.98 ± 0.05 | 6.58 ± 0.01 | 5.22 ± 0.01 | 3.21 ± 0.03 | −4.99 ± 0.03 | −8.01 ± 0.04 | −11.45 ± 0.33 | 37.89 ± 1.09 |
| CB | 23.23 ± 0.32 | 21.27 ± 0.20 | 17.06 ± 0.13 | 15.76 ± 0.14 | 12.79 ± 0.14 | 7.75 ± 0.12 | 2.62 ± 0.16 | 1.14 ± 0.15 | −1.35 ± 0.14 | 47.25 ± 3.29 |
- Note: Each value represents mean ± standard deviation of three replicates.
The initial crystallization for all samples started between 16.69 and 15.52°C. The second crystallization peak started between 6.99 and 6.51°C and the final crystallization peak initiated between −4.65 and −4.99°C and ended between −11.23 and −11.56°C. Previously reported crystallization onset temperatures varied between 14.64 and 17.00°C, which is comparable to the Dragon mango variety. However, the final offset temperature was higher (−11.23 to −11.56°C) compared to previous reports (−18.20 to −24.32°C) by Jin et al., (2017), Jahurul, et al., (2014a), Lieb et al., (2019), and Solis-Fuentes and Duran-de-Bazua (2004).
Crystallization starts with molecular rearrangement of highly saturated TAGs and ends with molecular aggregation and compaction (Tan & Che Man, 2002). The initial crystallization peak (T1) might be related to the high melting temperature saturated FA and TAGs which were observed in Tables 2 and 3. β crystals are the result of high levels of StOSt whereas POSt and POP are responsible for the formation of β′ crystals (Toro-Vazquez et al., 2004). Hence, the presence of high StOSt content in Dragon variety MKF may produce more β crystals which can also be associated with the higher melting endpoint (Table 4). The small peaks (T2 and T3) may be associated with the presence of unsaturated fatty acids and their associated TAGs (Figure 3). The temperature transitions observed in this study were in line with previous studies (Jahurul, et al., (2014a); Jin et al., 2017; Sonwai & Ponprachanuvut, 2014).
3.5 Solid fat content analysis
Solid fat content (SFC) for any fat can influence the final melting, storage, and sensory properties of a product (Lai & Lin, 2006). Figure 4 represents the SFC percentage of MKF at different temperatures. At 15–20°C, all the extracts contained 76%–87% SFC. All extracts started melting at around 25°C. Above 30°C, SFC declined abruptly. Below 25°C, the high content of SFC indicates the hardness of the product. High SFC at 25–30°C denotes the product's resistance to heating. High SFC at 30°C indicates that the product can be considered heat resistant (Torbica et al., 2015).
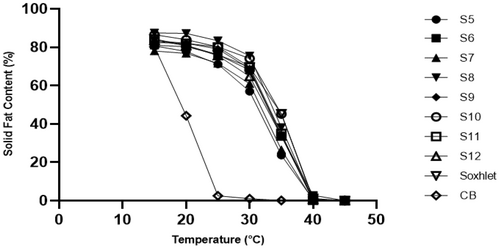
At 30°C, extracts S8 (T: 72°C, P: 30 MPa, 5 h) and S10 (T: 72°C, P: 25 MPa, 3 h) contained the highest SFC content (Figure 4). In previous studies, all crystallized fats in the MKF melted at 32–40°C (Kaphueakngam et al., 2009; Momeny et al., 2013; Solis-Fuentes & Duran-de-Bazua, 2004). In the current study, all the samples melted between 40 and 45°C which could probably be related to the higher percentages of StOSt content compared to previous studies (Table 3).
Torbica et al. (2015) reported an SFC of 78.11% in CB at 20°C, declining to 55.53% at 30°C and melting completely at 35°C. However, the amount of SFC at 35°C in cocoa butter improver (CBI) was reported as 32.19%. The Dragon variety of mango used in this study contained a higher SFC compared to commercial CB (Figure 4). This renders it potentially useful in temperate regions for blending or confectionery usage.
4 CONCLUSION
This study demonstrated the extraction yields of the Dragon variety MKF at different extraction conditions. The maximum yield (9.65% ± 1.10) was recorded at 60°C, 30 MPa pressure and 4 h of supercritical CO2 extraction. This yield was comparable with literature but was achieved at lower temperature and pressure, which indicated more efficient extraction with less energy consumption compared to previous studies. Supercritical fluid extraction is a green technology that is helpful for the extraction of MKF. However, using this technique is costly and requires expertise. The Dragon variety mango contained comparatively higher percentages of stearic acid and StOSt than Thai, Indian, and Kenyan variety mangoes. As the StOSt percentages were higher than POSt, this variety can be a valuable consideration for further interesterification and blending with other oils for confectionery use. Besides that, further analysis of crystal morphology and polymorphism studies may provide more insight for future industrial applications.
AUTHOR CONTRIBUTIONS
MANSURA MOKBUL: Conceptualization; formal analysis; investigation; methodology; software; validation; visualization; writing – original draft. Yuen Lin Cheow: Resources; writing – review and editing. Lee Fong Siow: Funding acquisition; resources; supervision; writing – review and editing.
ACKNOWLEDGMENTS
The authors would like to acknowledge their HDR scholarships and the School of Science, Monash University Malaysia for supporting the current research. The authors are grateful to Doreen Mavis Patrick and Mohd Syamil Abdul-Razak for SC-CO2, HPLC, and GC technical support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




