Potential of the cocoa shell to improve the quality properties of a burger-like meat product
Abstract
Cocoa shell powder (CSP) has a high nutritional value due to their fiber content and bio-compounds, including polyphenols with high antioxidant activity. In this study, CSP (0%, 1.5%, and 3.0%) was incorporated into a meat burger and its effect on the properties of the raw and cooked hamburger was evaluated. The CSP significantly increased the cooked hamburger’s fiber (0.13% to 0.93%–1.78%) and lipids (10.74% to 13.34%–13.42%) content; increased the hardness but with a better chewiness; a lower decrease in weight and volume loss was evidenced during cooking. CSP did not affect the shelf life of the raw burger compared with the control (8 days at 4°C). Neither did it affect the sensory traits of cooked burgers (5 days at 4°C). A slight reduction in Pseudomonas (0.4 Log CFU g-1) was observed with a CPS increase (p < 0.05). In conclusion, CPS is a potential new ingredient for healthy meat burgers.
Novelty Impact Statement
Incorporating an agro-industrial by-product such as cocoa shells in the formulation of hamburgers represents the possibility of fortifying with dietary fiber, polyphenols, and lipids (PUFA) found naturally in the cocoa shell. The process performed improves cooking properties and sensory properties without affecting shelf life. In addition, this technology can be incorporated into other processed meat products.
1 INTRODUCTION
Of the meat industry products, the hamburger and frankfurters are among the most accepted products by the consumers. However, it should be considered that the “image” they have is “unhealthy” in many sectors of the population (Fernández-López et al., 2021). The meat industry must quickly adapt to government plans to reduce obesity and offer consumers more beneficial foods for their health (Perrett, 2020).
In recent decades interest in fiber-rich foods has increased due to their beneficial effect on human health. In particular, high consumption of dietary fiber has been associated with a lower risk of the onset of cardiovascular diseases, diabetes, hypertension, and gastrointestinal diseases (Raninen et al., 2011), as well as stress reliever (Anderson et al., 2009). In addition, fibers are known to exhibit numerous biological activities related to gastrointestinal health (Jackson & Jewell, 2019).
In the last years, researchers have proposed the addition of fiber to meat products (Das et al., 2020). This can promote a balanced and healthy diet and provide some new functionality, improving the rheological properties and stability when fat is partially replaced (Mehta et al., 2015). This addition is very well accepted by a significant portion of consumers, including flexitarians, and many commercial products already incorporate different types of dietary fiber. And if there is also a perception that this fiber comes from sustainable valorization techniques, much better (García-Herrero et al., 2019). Different by-products have been studied for their possible incorporation, such as passion fruit albedo flour that showed improvements in emulsion stability, chewiness, and decreased weight loss during cooking in a tilapia meat product due to the emulsifying capacity of the fibers (dos Santos et al., 2021); bael pulp residue in goat meat nuggets (Das et al., 2015) and dragon fruit peel in chicken nuggets (Madane et al., 2020) significantly improved emulsion stability, cooking yield, decreased lipid peroxidation and microbial counts due to its richness in bioactive compounds such as phenolic compounds and dietary fiber; and cocoa pod husk improved the technological parameters and the emulsion stability, being a good substitute for starch in the formulation of frankfurters due to its high water-holding capacity (WHC) (Delgado-Ospina, Martuscelli, et al., 2021), among others.
Cocoa shell (CS) contains significant quantities of total dietary fiber (18.3% to 59.0% dry matter) and possess a high nutritional value owing to the presence of a variety of biocompounds, such as phenolic compounds, theobromine, and lipids (Delgado-Ospina, Di Mattia, et al., 2020; Delgado-Ospina, Lucas-González, et al., 2021; Lecumberri et al., 2007). In this context, the addition of CS fiber has been proposed as a fat replacer in chocolate muffins. It provided the muffins with higher moisture and a more tender and crumbly texture, and reduced signs of hardening during storage (Martínez-Cervera et al., 2011), and on the preparation of fresh and stored wheat bread it provided an initial softening effect, especially for CS at 6% of incorporation (Collar et al., 2009).
Since very few studies indicate the addition of cocoa shells into meat products, in this work, we focused the study on evaluating the effect of adding cocoa shell powder (CSP) on texture, cooking properties, microbiological, and sensory properties as a potential new ingredient for healthy meat hamburgers.
2 MATERIALS AND METHODS
2.1 Cocoa shell powder
The cocoa shell was obtained from samples of cacao Criollo (Cuatrecasas 13,377 [COL]) (Dorr, 2015) collected directly from a farm located in Valle del Cauca (Colombia), located in the western part of the country, 4°07′53.0′′ N latitude, 76°13′30.9′′ W longitude, altitude 975 masl. The cacao samples were roasted at 135°C for 15 min, and the cocoa shell was obtained after mechanically separating the nibs (Delgado-Ospina, Di Mattia, et al., 2020). The cocoa shell was ground in an impact mill (IKA MF 10.2, Staufen, Germany) and passed through a 0.5 mm pore size screen (Dp < 500 μm). The chemical, physico-chemical, and techno-functional properties of CSP were investigated in our previous study (Delgado-Ospina, Lucas-González, et al., 2021).
2.2 Burger elaboration process and treatments
Burgers were prepared at the IPOA Research Group pilot plant at the Miguel Hernández University, Orihuela, Spain following an industrial formulation. The ingredients were: beef (veal) meat (60.8%), pork meat (32.8%), black pepper (0.3%), salt (1.4%), and water (4.7%). According to previous validations, two different concentrations of CSP (0%, 1.5%, and 3.0%) were added to the burger formulation for the treatments. The meat of all the formulations was ground in a meat grinder (Advance, Rhino, Mexico) and mixed with the CSP and the other ingredients in a bowl, portions of 90 g were formed by compression in a manual burger maker machine (Oval shape 110 mm x 85 mm x 12 mm). They were cooked in an electric duo heat grill at 175°C for 4 min on both sides at the same time.
2.3 Proximate composition
The proximate composition was determined in burgers according to the following AOAC methods: lipid (AOAC 991.36), protein (AOAC 981.10), moisture (AOAC 925.45), ash (AOAC 923.03), and fiber (AOAC 985.29) (Horwitz, 2000).
2.4 Physicochemical and physical analyses
The pH of burgers was measured for direct penetration into meat using a penetration electrode (5232) connected to a pH-meter (model 507 Crison, Barcelona, Spain). Colorimetric analysis was performed using a CM-700d Spectrophotometer (Konica Minolta, Osaka, Japan), measured directly on the surface of the uncooked and cooked burgers, with the following settings (illuminant D65, observer 10°). The CIELab color coordinates (L*, a*, and b*), chroma , hue angle , and color difference were determined. AMSA Guidelines for meat color evaluation was used (AMSA, 2012; Sánchez-Zapata et al., 2011).
Texture profile analysis was performed out on uncooked and cooked burgers. A Texture Analyzer TA-XT2i (Stable Micro Systems, Surrey, England) was used. Before testing, the temperature of the samples was stabilized at room temperature for at least 30 min. Burger sections (30 mm wide and 30 mm long) were subjected to a 2-cycle compression to 75% deformation of their original height with a speed of 5 mm/s and activation force of 5 g. The force-time deformation curves were obtained and calculated the following attributes: Hardness, Adhesiveness, Springiness, Cohesiveness, Gumminess, Chewiness, and Resilience (Fernández-López et al., 2019).
2.5 Measurement of lipid oxidation: Thiobarbituric acid index
The extent of lipid oxidation was determined by measuring the TBARS-reacting substances in CSP and raw burgers by using the procedure described by Rosmini et al. (1996) and Sáyago-Ayerdi et al. (2009). In brief, 2.0 g of sample was homogenized with 16 ml of 10% trichloroacetic acid (TCA) in stir for 15 min. The sample was placed at rest for 30 min in an ice bath. Homogenized sample was filtered through Whatman qualitative filter paper (grade 1) into 25 ml Erlenmeyer flasks. Two ml of the filtered solution was mixed with 2 ml of 0.5% thiobarbituric acid (TBA) in distilled water in capped test tubes. Tubes were incubated in boiling water for 35 min. The absorbance was determined at 532 nm against a blank containing 2 ml of 10% TCA and 2 ml of 0.5% TBA solution. Values were expressed as mg of malondialdehyde (MDA)/kg of the sample.
2.6 Fatty acid profile
For the fatty acid profile determination, the extraction of the lipids present in the CSP and burgers was carried using a mixture of chloroform: methanol (2:1 v/v). Following the lipid extracts (without solvent) were transmethylated with methanol and analyzed on a Gas Chromatography (Agilent, model 6890) equipped with a flame ionization detector (FID) and a Suprawax-280 capillary column (30 m length, 0.25 μm film, 0.25 mm internal diameter; Teknokroma, Barcelona, Spain) according to Lucas-González et al. (2020) (Limit of quantification 0.01 mg/g). The results were expressed as mg/g of fat.
2.7 Microbiological analyses
Ten grams of burger samples were added in 90 ml sterile saline solution and homogenized in a Stomacher Lab-blender. Decimal dilutions of the suspension were prepared in physiological solution, plated and incubated as follows: Mesophilic aerobic bacteria in Plate Count Agar (PCA) (Biolife, Milan, Italy) at 30°C for 48 h; Psychrotrophic aerobic bacteria in PCA and incubated at 8°C for 7 days; Lactic Acid Bacteria in Lactobacillus Agar according to DeMan, Rogosa and Sharpe (MRS) (Oxoid, Basingstoke, UK) at 37°C in anaerobiosis for 72 h; Staphylococcus aureus and coagulase-negative Staphylococcus in Baird-Parker Agar added with Egg Yolk Tellurite Emulsion (Oxoid, Basingstoke, UK) at 37°C for 48 h; yeasts in Peptone Yeast Extract agar (YPD) and Wallertstein Laboratory Nutrient Medium (WL agar) (Biolife, Milan, Italy) at 25°C for 48 h; molds in Dichloran Glycerol Agar (DG18) (Oxoid, Basingstoke, UK) and Czapec Dox agar (Sigma-Aldrich, Milan, IT) for 5 days; Enterobacteriaceae and total coliforms were counted and isolated in Violet Red Bile Glucose Agar and Violet Red Bile Agar (Oxoid, Basingstoke, UK) at 37°C for 24 h respectively in anaerobiosis; Pseudomonas spp. were enumerated on cetrimide fucidin cephaloridine agar (Liofilchem, Teramo, IT) at 25°C for 48 h. The visible colony count at the end of the incubation period and the dilution factor were used to determine the number of microorganisms present in the sample. Presumptive Clostridium sulfite reducing was searched by the Most Probable Number method using Reinforced Clostridium Broth (Biolife, Milan, Italy) incubated in anaerobiosis at 37°C for 48 h. The results were expressed as Log CFU/g sample.
2.8 Cooking properties
2.9 Volatiles compounds analysis
The evolution of the volatiles compounds profile was investigated during the refrigerated storage at a different time (0, 72, and 120 h). Once the storage time had elapsed, the samples were cooked and were immediately put in glass vials of 20 ml capacity (Perkin Elmer) with approximately 3.0 g of meat finely chopped, assuring the highest headspace, tightly closed and stocked at −40°C until analysis. For the GC–MS analysis, the method used was taken from Qi et al. (2018) with some modifications. Vials stocked were left for 1 h out of the freezer at room temperature, then put in a water bath at 50°C for 20 min.
Volatiles from meat were extracted with a headspace solid phase microextraction fiber (65 μm Polydimethylsiloxane/Divinylbenzene -PDMS/DVB-; Supelco, Bellofonte, USA) and collected for 30 min at 40°C. The fiber was then inserted into the GC/Mass Spectrometer injector (Clarus SQ 8S, Perkin Elmer, Waltham, Massachusetts, USA) and desorbed for 3 min at 250°C. Volatile compounds were separated on a Capillary GC column ZB- Semi Volatiles (30 m length, 0.25 mm internal diameter, 0.25 μm film thickness) (Phenomenex, USA). The oven temperature was maintained for 3 min at 40°C, increased at 3°C/min to 70°C, then 5°C/min to 180°C, then at 10°C/min to 260°C, and maintained for 5 min at 260°C. Helium was the carrier gas with a constant flow of 1 ml/min. The mass-selective detector was operated in the electron impact mode (70 eV) and full scan mode (35–500 m/z range).
2.10 Sensory evaluation
The burgers were tested for the intensity of flavor attributes on a scale of 0 (=absent) to 5 in order to evaluate odor, flavor, and taste properties. Sensory analysis was carried out by a group of nine panelists trained (five women and four men) for evaluation of the quality assessment of meat burgers, according to protocols described by Longato et al. (2017); the selection of descriptors was done on the list of sensory terms defined by Byrne et al. (2002). The panel had no background information about the samples.
Experimental samples were evaluated in a total of nine sessions held over 3 days (0, 72, and 120 h, with 3 sessions/day, with a 20-min break between sessions). The three coded samples were served in a white dish on each evaluation session, evaluating only the samples of the corresponding day. Each panelist evaluated three replicates of all burger samples; tap water was provided to cleanse the palate. Panelists evaluated four classes of descriptors: odor (cooked meat, cardboard-like, sulfur/rubber, roasty, painty), flavor (cooked meat, rancid-like), taste (vegetable oil-like, sour, bitter), and after taste (metallic, astringent).
Sensory evaluation was carried out on meat burgers immediately after preparation (raw samples), after cooking (0 h), and after refrigerated storage (4°C, until to 120 h) of cooked samples. For the sensory test at 72 h, burger’ samples were re-heated in a hot water bath until reaching the core temperature of 60°C–62°C, as suggested by Byrne et al. (2002). A sniffing test was also developed on burgers kept at 4°C for 120 h. These samples were not eaten to assure the safety of the people involved.
2.11 Statistical analysis
Three independent experiments were made, three replications of each factor and level were made, and three repeats were analyzed for each sample. The one-way analysis of variance (ANOVA) was carried out to evaluate the statistical significance (p < 0.05) of the treatments. The means comparisons were made using the Tukey HSD test (p ≤ 0.05). All data are presented as mean values ± standard deviation (SD). The Statgraphics Centurion XVI program was used for these statistical analyses.
3 RESULTS AND DISCUSSION
3.1 Proximate composition of burger
The compositional analysis of the CSP was previously reported by us (Delgado-Ospina, Lucas-González, et al., 2021). The addition of the CSP to the burger did not show significant differences (p < 0.05) in the pH. Although the pH of CSP is slightly lower (5.34 ± 0.02), it was not able to lower the pH of the burger, thus avoiding a greater susceptibility of muscle pigments to oxygenation and oxidation and, consequently, the formation of higher amounts of metmyoglobin that change the color of the meat. Similarly, the protein content did not change.
As expected, the addition of CSP increased the burger’s fiber content (Table 1). The increase in fiber content was within the expected value according to the addition made. Increases in DF have been reported in chicken nuggets of up to 2.37%, with additions of 3% of dragon fruit peel (Madane et al., 2020) and of 3.5% in sheep meat nuggets with the addition of 1% of guava powder (Verma et al., 2013). Although the value obtained is low, the results indicate that burgers became nutritionally enriched due to the inclusion of DF. A burger (100 g, 290 cal) added with cocoa fiber (CSP 3.0%) could provide 1.78 g of DF, which corresponds to 7% of the daily fiber needs.
| Raw burger | Cooked burger | |||||
|---|---|---|---|---|---|---|
| Control | CSP1.5% | CSP3.0% | Control | CSP1.5% | CSP3.0% | |
| Protein (%) | 18.87 ± 0.12 a | 18.61 ± 0.66 a | 18.41 ± 0.28 a | 27.23 ± 1.06 b | 25.51 ± 0.63 b | 25.79 ± 0.01 b |
| Lipid (%) | 7.17 ± 1.08 a | 8.30 ± 1.62 a | 10.54 ± 0.97 b | 10.74 ± 1.21 b | 13.34 ± 0.97 c | 13.42 ± 0.49 c |
| Crude fiber (%) | 0.03 ± 0.02 a | 0.76 ± 0.12 b | 1.35 ± 0.23 c | 0.13 ± 0.10 a | 0.93 ± 0.22 b | 1.78 ± 0.35 d |
| Moisture (%) | 67.21 ± 0.1 d | 65.73 ± 1.0 c | 64.96 ± 0.4 c | 56.12 ± 0.4 b | 55.97 ± 0.3 b | 54.35 ± 0.4 a |
| Ash (%) | 2.41 ± 0.03 b | 2.54 ± 0.04 c | 2.27 ± 0.01 a | 3.11 ± 0.03 e | 3.12 ± 0.01 e | 3.00 ± 0.01 d |
| pH | 5.71 ± 0.02 a | 5.69 ± 0.01 a | 5.69 ± 0.01 a | 6.06 ± 0.02 b | 6.04 ± 0.01 b | 6.06 ± 0.01 b |
| aw | 0.949 ± 0.003 b | 0.955 ± 0.002 c | 0.951 ± 0.004 bc | 0.947 ± 0.004 b | 0.943 ± 0.002 a | 0.940 ± 0.002 a |
| TBA* (mg MDA/kg product) | 0.33 ± 0.05 a | 0.44 ± 0.10 ab | 0.62 ± 0.06 b | |||
- * TBA, thiobarbituric acid value; MDA, malonaldehyde. Results are expressed as means of three samples ± SD. Values followed by the same small letter within the same row are not significantly different (p > 0.05) according to Tukey’s multiple-range test.
In addition, an increase in lipid content was also observed for both treatments. This increase is related to the contribution of lipids from the CSP and mainly by the oil-holding capacity (OHC) of the CSP that prevents the loss of lipids or volatile organic compounds during its determination. In the determination (AOAC 991.36), the samples are heated at 125°C for 1 h to eliminate the water; when the fiber is present, it retains some volatile lipids, so a decrease in the moisture content is observed (less loss in the determination moisture) and in parallel an increase in lipid content (Pietrasik et al., 2020). Moisture determinations by the gravimetric method are subject to a margin of error due to the evaporation of volatile compounds naturally present in the samples.
It is well known that the increase in fiber and fat content from some vegetable sources high in unsaturated fatty acids improves the product’s nutritional characteristics (Fernández-López et al., 2019). TBARs values in all treatments were below the level of incipient rancidity (≥1.0). However, a significant increase (p < 0.05) of this parameter was observed with the CSP concentration incorporated into the burger; this increase may be related to highly unsaturated fatty acids present in the CSP. As regards the humidity, a slight reduction was detected with the increase of CSP because the fiber absorbs water from the medium, even when the fiber has a low WHC (4.62 g H2O/g CSP) (Delgado-Ospina, Lucas-González, et al., 2021), which will be reflected in the texture properties of the burger. In general, CSP contains 70% in insoluble fiber; this increase in insoluble fiber with its low water retention can cause a rough sensation in the mouth of meat products (Zhao et al., 2018), which can be avoided by reducing the size of the added fiber.
After cooking, an expected significant increase (p < 0.05) of the fiber was observed, correlated to the humidity’s decrease. The moisture was reduced significantly in 3.0% CSP samples due to the major incorporation of the CSP in this treatment. The lipids showed a significant increase (p < 0.05) concerning the control, but not among the treatments; this increase is related to the contribution of lipids from the CSP and their OHC (1.30 g/g CSP) that prevents lipids’ loss during the cooking process. Although the protein content appeared to be reduced, this reduction was not statistically significant (p > 0.05); this decrease is directly related to the final product’s increase in fiber and lipid content. The pH did not show significant changes.
3.2 Fatty acid profile
The main fatty acids found in the CSP were palmitic acid (C16:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3), similar with those reported by (Okiyama et al., 2019) (Table 2). These fatty acids come mainly from the beans due to migration during fermentation, drying, and especially roasting (Agus et al., 2018), where high temperatures favor migration. Additionally, the cocoa shell contains small fractions of beans that are dragged along with the CSP (Delgado-Ospina, Lucas-González, et al., 2021), so the fatty acid profile of CSP is similar to that of cocoa butter (Okiyama et al., 2019). For this reason, it has been considered a promising source to obtain cocoa butter for different applications, mainly in the confectionery industry; due to the composition of its crystal lattice confers to the product appropriate physical properties how brightness, brittleness, and melting properties (Lipp et al., 2001).
| Raw burger | Cooked burger | ||||||
|---|---|---|---|---|---|---|---|
| Cocoa Shell | Control | CSP1.5% | CSP3.0% | Control | CSP1.5% | CSP3.0% | |
| C10:0 | 0.00 ± 0.00 | 0.75 ± 0.07 mA | 0.73 ± 0.04kA | 0.65 ± 0.07 nA | 0.69 ± 0.07kA | 0.67 ± 0.21 mA | 0.65 ± 0.12kA |
| C12:0 | 0.25 ± 0.07j | 0.80 ± 0.00mC | 0.75 ± 0.07kB | 0.85 ± 0.07 nA | 0.80 ± 0.014kA | 0.83 ± 0.17 mA | 0.80 ± 0.34jkA |
| C14:0 | 1.65 ± 0.21 h | 18.45 ± 0.07fA | 16.60 ± 0.14fC | 17.80 ± 0.14fB | 17.20 ± 0.21fA | 17.50 ± 0.26fA | 17.35 ± 0.37fA |
| C14:1 | 0.00 ± 0.00 | 2.65 ± 0.07jA | 2.30 ± 0.14iB | 2.35 ± 0.07mB | 2.33 ± 0.28jA | 2.34 ± 0.35kA | 2.15 ± 0.24iA |
| C15:0 | 0.00 ± 0.00 | 1.50 ± 0.00kB | 1.35 ± 0.21jB | 6.15 ± 0.61hA | 3.75 ± 0.28hA | 4.95 ± 0.44iA | 1.25 ± 0.36jB |
| C15:1 | 0.00 ± 0.00 | 0.45 ± 0.07mB | 0.55 ± 0.07mB | 5.45 ± 0.07iA | 3.00 ± 0.14iB | 4.23 ± 0.38iA | 0.00 ± 0.00 |
| C16:0 | 271.45 ± 0.49c | 238.30 ± 0.28bA | 242.70 ± 0.85bA | 239.95 ± 0.21bA | 241.33 ± 0.07bB | 240.64 ± 0.65B | 249.40 ± 0.41bA |
| C16:1 | 4.75 ± 0.49f | 28.20 ± 0.28eA | 28.05 ± 0.35eA | 27.60 ± 0.57eA | 27.83 ± 0.14eB | 27.71 ± 0.21B | 36.35 ± 0.38eA |
| C17:0 | 2.75 ± 0.35 g | 4.20 ± 0.14 hA | 3.90 ± 0.14 hB | 4.00 ± 0.14jAB | 3.95 ± 0.35hA | 3.98 ± 0.74jA | 3.75 ± 0.36hA |
| C17:1 | 0.00 ± 0.00 | 3.65 ± 0.07iA | 3.40 ± 0.14 hB | 3.20 ± 0.28kB | 3.30 ± 0.18iA | 3.25 ± 0.69jB | 3.30 ± 0.58hA |
| C18:0 | 314.50 ± 0.71b | 135.59 ± 0.54cC | 141.85 ± 0.92cA | 137.15 ± 0.07cB | 139.50 ± 0.17cB | 138.33 ± 0.36cC | 145.25 ± 1.25cA |
| C18:1 | 344.90 ± 0.57a | 461.95 ± 0.21aA | 448.95 ± 1.34aB | 435.70 ± 0.28aC | 442.33 ± 0.16aA | 439.01 ± 0.34aB | 421.00 ± 1.25aC |
| C18:2 | 41.75 ± 0.49d | 98.25 ± 0.35dA | 94.05 ± 0.64 dB | 100.40 ± 0.99dA | 97.23 ± 0.27dC | 98.81 ± 0.29 dB | 104.35 ± 0.74dA |
| C18:3 (n3,6,9) | 2.75 ± 0.21 g | 0.00 ± 0.00 | 6.40 ± 0.42gB | 8.70 ± 0.28gA | 7.55 ± 0.54gA | 8.13 ± 0.23gA | 5.70 ± 0.87gB |
| C20:0 | 10.75 ± 0.21e | 5.25 ± 0.07gA | 2.25 ± 0.07iC | 3.10 ± 0.14kB | 2.68 ± 0.28jA | 2.89 ± 0.74kA | 2.10 ± 0.36iB |
| C20:1 | 0.70 ± 0.14i | 0.00 ± 0.00 | 6.15 ± 0.21 g | 6.95 ± 0.41 h | 6.55 ± 0.71gA | 6.75 ± 0.36hA | 6.60 ± 0.24gA |
| C22:0 | 2.55 ± 0.14 g | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| C24:0 | 1.14 ± 0.14 h | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| ΣSFA | 605.13 ± 2.02 | 404.09 ± 2.15B | 410.13 ± 1.63A | 409.65 ± 1.47A | 417.10 ± 1.25A | 406.23 ± 2.36B | 420.55 ± 2.78A |
| ΣMUFA | 350.3 ± 1.28 | 496.90 ± 1.85A | 489.40 ± 1.78B | 481.25 ± 1.68C | 481.55 ± 2.43A | 483.45 ± 2.74A | 469.40 ± 1.98B |
| ΣPUFA | 44.52 ± 1.41 | 99.01 ± 1.23C | 100.47 ± 1.45B | 109.10 ± 1.33A | 110.35 ± 1.78A | 110.32 ± 1.11A | 110.05 ± 1.39A |
- Notes: Values expressed as mg/g of fat. Results are expressed as means of three samples ± SD. For each group (raw or cooked) values followed by the same capital letter within the same row are not significantly different (p > 0.05) according to Tukey’s multiple-range test. Values followed by the same small letters within the same column are not significantly different (p > 0.05) according to Tukey’s multiple-range test.
Incorporating CSP to the hamburger provides fatty acids of higher-molecular weight (C16 to C24), many of which are not found in the control burger lipid profile, such as C18:3 (n3,6,9), which can be beneficial to the health of consumers. In general, the addition of CSP1.5% and CSP3.0% increases the concentration of PUFA, which may have benefits against some diseases (Hernandez-Rodas et al., 2016). A significance decrease in MUFA (496.90 to 481.25 mg/g of fat) was also observed due to the lower contribution of the CSP (350.3 mg/g of fat) and an increase in the SFA (404.09 to 410.13 mg/g of fat) in the burgers due to the higher contribution of the CSP (605.13 mg/g of fat).
A lipid profile similar to raw burgers was found in cooked burgers. The small changes found can be attributed to a thermo-oxidative effect during heating (Żyżelewicz et al., 2014).
3.3 Color
Color is the main quality attribute consumers consider when selecting a processed meat product. The CSP has a distinctive brown color that can change the products' color to which can be added. The dried CSP showed an L* value of 51.49 ± 0.04, which decreased to 35.39 ± 0.52 when subjected to hydration, lower than the burger without CSP addition (Table 3). The addition of the CSP to the burger caused the decrease of L* values. This effect can be attributed to two factors. The first is the contribution of the dark color of the CSP hydrated inside the matrix. The second is the light reflection phenomena in the burger’s surface, mainly due to the decrease “in free water in the surface” and moisture content in the samples caused by the high WHC that the CSP possesses.
| L* | a* | b* | C* | h ab | ΔE* | |
|---|---|---|---|---|---|---|
| CSP | ||||||
| Dry | 51.49 ± 0.04 | 10.62 ± 0.01 | 19.32 ± 0.01 | 22.05 ± 0.01 | 61.21 ± 0.03 | |
| Hydrated | 35.39 ± 0.52 | 8.09 ± 0.28 | 10.03 ± 0.41 | 12.88 ± 0.50 | 51.09 ± 0.18 | |
| Raw burger | ||||||
| Control | 46.94 ± 0.17 b | 13.64 ± 0.11 d | 20.26 ± 0.02 e | 24.42 ± 0.05 e | 56.04 ± 0.24 a | – |
| CSP1.5% | 43.39 ± 1.38 a | 10.19 ± 0.25 c | 17.91 ± 0.73 c | 20.61 ± 0.52 c | 60.34 ± 1.55 b | 7.82 ± 3.17 a |
| CSP3.0% | 41.94 ± 1.0 a | 9.75 ± 0.11 c | 19.32 ± 0.30 d | 21.64 ± 0.21 d | 63.21 ± 0.62 b | 10.35 ± 1.37 a |
| Cooked burger | ||||||
| Control | 53.33 ± 1.03 d | 5.32 ± 0.73 ab | 13.12 ± 0.76 a | 14.18 ± 0.70 a | 67.91 ± 3.21 c | – |
| CSP1.5% | 49.07 ± 1.38 c | 5.24 ± 0.53 a | 13.90 ± 0.32 ab | 14.86 ± 0.35 ab | 69.35 ± 1.96 c | 9.45 ± 1.77 a |
| CSP3.0% | 47.52 ± 1.11 bc | 5.96 ± 0.33 b | 14.35 ± 0.35 b | 15.54 ± 0.38 b | 67.44 ± 1.07 c | 11.47 ± 1.36 a |
- Notes: L*, lightness; a*, red/green coordinate; b*, yellow/blue coordinate; C*, Chrome; hab hue angle; ΔE, color differences. Results are expressed as means of four samples ± SD. Different letters in the same column indicate significant differences (p < 0.05) according to Tukey’s multiple-range test.
On the other hand, a* and b* values are helpful to identify the evolution of a meat product, both decrease during oxidation, being the best indicators of metmyoglobin changes during oxidation (Hernández Salueña et al., 2019). The addition of CSP caused a significant decrease in values a* and b* in the burger samples. Although this tendency may suggest possible oxidation of metmyoglobin, the short time between the addition of CSP and the measurement of the a* and b* parameters indicated that the changes are related to the CSP color’s contribution and not oxidative processes.
Chroma C* and hue angle h* directly correlate with human visual color perception. The increase of CSP in burgers decreased C* values and an increase in hab values, so the samples showed a lower vividness of color, and the tone shifted from red to yellow. According to (Hernández Salueña et al., 2019), the oxidation of metmyoglobin in meat implies a decrease in C* values but not a significant change in hab, reinforcing the argument that color changes are related to the color contribution of the CSP.
The ΔE* is an excellent parameter to track color changes if it is established the threshold is at which an observer evaluates a sample as different. Although values for meat products like burger meat have not been established, most reports indicate that values >3 are perceptible changes by the observer (Fernández-López et al., 2019). In this sense, CSP addition gives a different color to the samples with relation to the control sample, increasing as the concentration of the CSP increases.
In cooked burgers added with CSP, the color change evaluated as ΔE* presented values greater than 3, which indicates that the samples are significantly different from the control, but not between them (p < 0.01). It is possible that the color difference already observable for the consumer between the control and the samples added with CSP can generate discrepancies on the appropriate cooking time (Figure 1). The significant decrease in luminosity concerning the control is due to the same factors mentioned above for adding CSP to meat; lower luminosity implies a darker color that can be confused with early cooking or an excess of cooking. The increase of b* and the Chroma may reflect lower oxidation of the metmyoglobin concerning the control (Hernández Salueña et al., 2019) due to increased temperature.
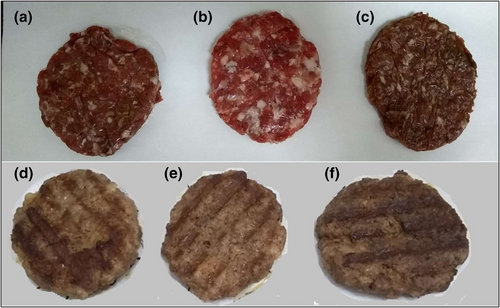
3.4 Texture profile analysis
The texture profile analysis (TPA) performed on raw burgers (Table 4) showed that in most of the parameters, there was a significant variation (p < 0.05). At this point, the texture parameters impact their shelf life and the initial consumer perception. In raw burger, hardness increased with the addition of CSP (p < 0.05) in accordance with Sánchez-Zapata et al. (2013), who found that the addition of insoluble fiber to sausages increased their hardness, attributed to the ability of some fibers to promote or strengthen connections among the matrix components (Cruz et al., 2010). This is favorable because the addition of binders agents such as wheat crumb (Pietrasik et al., 2020) or breadcrumbs and egg (Sáyago-Ayerdi et al., 2009) can be avoided to maintain the desired shape.
| Hardness (N) | Adhesiveness | Springiness (mm) | Cohesiveness | Gumminess (N) | Chewiness (N mm) | Resilience | |
|---|---|---|---|---|---|---|---|
| Raw burger | |||||||
| Control | 57.59 ± 2.29 a | 1.93 ± 0.04 b | 0.30 ± 0.02 c | 0.39 ± 0.01 c | 22.60 ± 1.09 b | 6.82 ± 1.11 b | 0.126 ± 0.007 b |
| CSP1.5% | 58.24 ± 2.73 ab | 2.09 ± 0.66 b | 0.23 ± 0.06 b | 0.33 ± 0.02 a | 19.82 ± 1.54 a | 4.52 ± 1.23 a | 0.097 ± 0.007 a |
| CSP3.0% | 62.95 ± 1.05 b | 3.10 ± 1.49 b | 0.23 ± 0.03 b | 0.36 ± 0.02 b | 22.86 ± 0.96 b | 5.36 ± 0.75 a | 0.103 ± 0.07 a |
| Cooked burger | |||||||
| Control | 184.50 ± 76.11 c | 0.010 ± 0.009 a | 0.19 ± 0.06 b | 0.67 ± 0.02 e | 123.37 ± 19.80 c | 21.65 ± 0.74 d | 0.274 ± 0.015 d |
| CSP1.5% | 249.13 ± 45.97 d | 0.011 ± 0.009 a | 0.12 ± 0.02 a | 0.67 ± 0.01 e | 166.25 ± 32.58 d | 19.82 ± 0.50 c | 0.267 ± 0.015 d |
| CSP3.0% | 236.56 ± 19.75 cd | 0.012 ± 0.003 a | 0.13 ± 0.02 a | 0.65 ± 0.01 d | 153.54 ± 12.44 d | 19.55 ± 1.01 c | 0.253 ± 0.010 c |
- Notes: Results are expressed as means of four samples ± SD. Different letters in the same column indicate significant differences (p < 0.05) according to Tukey’s Multiple Range Test.
Adhesiveness remained constant while springiness, cohesiveness, gumminess, chewiness, and resilience decreased (p < 0.05) when the CSP was added. The decrease in these parameters may be related to the decrease in burger hydration per effect high fiber WHC.
In cooked burgers, the hardness increased significantly (p < 0.05). It is important to underline that this value was further increased with the cooking. The insoluble polysaccharides of the CSP probably participate in a thermally activated insoluble three-dimensional network (Sánchez-Zapata et al., 2013). This can occur through interaction with water molecules by capillarity, hydrogen bonds, ionic interactions with polar groups of proteins, or within the matrix (Cava et al., 2012). At this point, the networks protein–water, protein–protein interaction, or new interactions between CSP and proteins, increase the gel strength. The insoluble fiber favors the fixing of water and the absorption of fats, increasing the stability of the emulsions. In general, an increase in fat content in the product generate a decrease in hardness; in our case, it was not observed due to the strong effect of fiber interaction.
The gumminess also increased significantly with the addition of CSP without significant differences between the two CSP concentrations. Adhesiveness and cohesiveness remained constant while springiness, chewiness, and resilience decreased (p < 0.05) in samples with CSP addition. The decrease in these parameters may be related to reducing burger hydration by adding fiber. In general, the product was presented with a greater hardness but with a better chewiness.
3.5 Microbiological counts
It is well known that CSP harbored a particular microbiota deriving from the fermentation and drying process, in particular fungi (Delgado-Ospina, Molina-Hernández, et al., 2021), yeasts (Delgado-Ospina, Triboletti, et al., 2020), lactic acid bacteria, and acetic acid bacteria, as well as some microorganisms from cross-contamination during the process (Delgado-Ospina, Di Mattia, et al., 2020; Schwan & Wheals, 2004). However, during the roasting process, most of these microorganisms are eliminated.
As evidenced in Table 5, the CSP microbiota was represented by lactic acid bacteria, fungi, and yeasts found naturally in cocoa. It has been shown that some yeasts found in fermented and dried cocoa beans can be acid-, osmo-, thermo-, and desiccation-tolerant and that this dependence is closely related to specific substrates such as polyphenols in cocoa (Delgado-Ospina, Triboletti, et al., 2020). Additionally, there were low counts in Pseudomonas and coliforms (2.8 and 2.4 log CFU/g, respectively). In general, the mesophilic aerobic bacteria found was 4.8 log CFU/g, of which a small percentage of these can survive at a temperature of 10°C. Neither Staphylococcus sp. nor sulfite reducing clostridial and Salmonella sp. were found in the different samples analyzed.
| Storage time (days) | ||||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | ||
| Mesophilic aerobic bacteria | CSP | 4.8 ± 0.1 | ||||
| Control | 3.5 ± 0.1 aA | 3.8 ± 0.1 aB | 4.5 ± 0.1 aC | 5.0 ± 0.2 bD | 5.8 ± 0.4 bE | |
| CSP1.5% | 3.8 ± 0.1 bA | 4.1 ± 0.2 bB | 4.3 ± 0.2 aC | 4.6 ± 0.2 aC | 5.0 ± 0.3 aD | |
| CSP3.0% | 4.3 ± 0.1 cA | 4.7 ± 0.1 cB | 5.0 ± 0.2 bC | 5.1 ± 0.1 bC | 5.1 ± 0.3 aC | |
| Psychrotrophic bacteria (10°C) | CSP | 3.8 ± 0.1 | ||||
| Control | 4.0 ± 0.1 aA | 3.9 ± 0.2 aA | 5.4 ± 0.3 bB | 5.9 ± 0.2 cC | 6.7 ± 0.4 dD | |
| CSP1.5% | 3.9 ± 0.2 aA | 3.9 ± 0.2 aA | 5.2 ± 0.4 bB | 6.0 ± 0.3 cC | 6.6 ± 0.3 dD | |
| CSP3.0% | 3.9 ± 0.2 aA | 3.9 ± 0.1 aA | 5.4 ± 0.3 bB | 6.0 ± 0.3 cC | 6.7 ± 0.4 dD | |
| Mold | CSP | 3.1 ± 0.2 | ||||
| Control | n.d. | n.d. | n.d. | n.d. | n.d. | |
| CSP1.5% | n.d. | n.d. | n.d. | n.d. | n.d. | |
| CSP3.0% | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Yeast | CSP | 3.3 ± 0.1 | ||||
| Control | n.d. | 1.5 ± 0.2 aA | 2.7 ± 0.2 aB | 3.0 ± 0.3 aC | 3.2 ± 0.2 aC | |
| CSP1.5% | n.d. | 1.7 ± 0.3 abA | 2.8 ± 0.1 aB | 3.2 ± 0.2 abC | 3.5 ± 0.1 bD | |
| CSP3.0% | n.d. | 1.9 ± 0.3 bA | 3.1 ± 0.1 bB | 3.3 ± 0.2 bC | 3.5 ± 0.1 bC | |
| Enterobacteriaceae | CSP | n.d. | ||||
| Control | 2.0 ± 0.1 aA | 2.3 ± 0.1 aB | 3.0 ± 0.1 aC | 3.2 ± 0.1 aD | 3.2 ± 0.1 aD | |
| CSP1.5% | 2.1 ± 0.1 aA | 2.4 ± 0.2 aB | 3.0 ± 0.2 aC | 3.2 ± 0.1 aC | 3.2 ± 0.2 aC | |
| CSP3.0% | 2.2 ± 0.1 aA | 2.3 ± 0.1 aA | 3.1 ± 0.2 aB | 3.2 ± 0.1 aB | 3.1 ± 0.2 aB | |
| Total coliform | CSP | 2.4 ± 0.1 | ||||
| Control | 2.5 ± 0.1 aA | 2.8 ± 0.1 aB | 3.1 ± 0.2 aC | 3.6 ± 0.2 aD | 3.5 ± 0.1 aD | |
| CSP1.5% | 2.5 ± 0.1 aA | 2.8 ± 0.2 aB | 3.1 ± 0.1 aC | 3.4 ± 0.2 aD | 3.5 ± 0.2 aD | |
| CSP3.0% | 2.6 ± 0.1 aA | 2.8 ± 0.1 aB | 3.0 ± 0.2 aC | 3.4 ± 0.1 aD | 3.5 ± 0.2 aD | |
| Lactic acid bacteria | CSP | 4.5 ± 0.2 | ||||
| Control | 3.5 ± 0.2 aA | 3.6 ± 0.1 aAB | 3.8 ± 0.2 aB | 4.1 ± 0.2 aC | 4.1 ± 0.2 aC | |
| CSP1.5% | 3.6 ± 0.2 aA | 3.7 ± 0.1 aA | 3.8 ± 0.1 aA | 3.9 ± 0.2 aB | 4.1 ± 0.1 aB | |
| CSP3.0% | 3.6 ± 0.2 aA | 3.7 ± 0.2 aA | 3.7 ± 0.2 aA | 3.9 ± 0.1 aB | 4.0 ± 0.1 aB | |
| Pseudomonas sp | CSP | 1.8 ± 0.1 | ||||
| Control | 2.1 ± 0.1 bA | 2.1 ± 0.2 bA | 2.8 ± 0.1 bB | 3.2 ± 0.2 bC | 3.8 ± 0.2 bD | |
| CSP1.5% | 1.7 ± 0.1 aA | 1.8 ± 0.1 aA | 2.1 ± 0.1 aB | 2.5 ± 0.2 aC | 3.1 ± 0.2 aD | |
| CSP3.0% | 1.7 ± 0.1 aA | 1.8 ± 0.2 aAB | 2.0 ± 0.1 aB | 2.5 ± 0.2 aC | 3.0 ± 0.2 aD | |
| Staphylococcus sp | CSP | n.d. | ||||
| Control | 2.8 ± 0.2 aA | 4.7 ± 0.3 aB | 5.9 ± 0.2 aC | 6.0 ± 0.2 aC | 6.1 ± 0.4 aC | |
| CSP1.5% | 2.6 ± 0.2 aA | 4.7 ± 0.4 aB | 5.8 ± 0.3 aC | 5.9 ± 0.2 aC | 6.0 ± 0.3 aC | |
| CSP3.0% | 2.6 ± 0.3 aA | 4.8 ± 0.3 aB | 5.8 ± 0.3 aC | 5.8 ± 0.3 aC | 6.0 ± 0.4 aC | |
| Sulfite-reducing Clostridia | CSP | n.d. | ||||
| Control | n.d. | n.d. | n.d. | n.d. | n.d. | |
| CSP1.5% | n.d. | n.d. | n.d. | n.d. | n.d. | |
| CSP3.0% | n.d. | n.d. | n.d. | n.d. | n.d. | |
- Notes: Results are expressed as means of three samples ± SD. Values expressed as Log CFU/g sample. For each test or microorganism group: values followed by the same capital letter within the same row (treatments) are not significantly different (p > 0.05) and values with different small letters within the same column (day of storage) are not significantly different (p > 0.05) according to Tukey’s multiple-range test.
As observed in Table 5, except for a slight increase (0.8 Log CFU/g) in mesophilic bacteria with the addition of CSP3.0%, the incorporation of CSP in burger did not lead to a significant increase of bacterial groups here studied. On the contrary, a reduction in Pseudomonas (0.4 Log CFU/g) with the increase in CSP (p < 0.05) was observed, probably small changes presented in pH, osmolarity, and the presence of metabolites such as polyphenols could influence the decrease of this microbial group.
During refrigerated storage at 4°C, mesophilic bacteria and Pseudomonas growth were restricted in samples added with CSP concerning the control ones; this result is particularly interesting. In particular, at the end of the storage (8 days), mesophilic aerobic bacteria in control samples were about 0.8 CFU/g higher than in samples with CSP, this reduction was associated with the reduction in Pseudomonas, which was also reduced by 0.8 Log CFU/g in samples added with CSP. This result is particularly interesting since Pseudomonas spp. is one of the most common spoilage bacteria in refrigerated meat (Paparella et al., 2016).
In general, the incorporation of CSP in burgers despite the initial content of microorganisms did not affect the burger’s shelf life concerning the control. On the contrary, the addition of CSP favored the decrease in Pseudomonas, probably due to the presence of polyphenols (9.53 mg GAE/g) in CSP (Delgado-Ospina, Lucas-González, et al., 2021). In this regard, Santos et al. (2014) reported the antimicrobial effects of cacao pod husks against Pseudomonas aeruginosa; on the other hand (Chaves-López et al., 2018) reported the efficacy of the polyphenols luteolin and myricetin, and in less extend catechin, singly tested, to reduce the population in P. aureuginosa.
It was shown that the SC microbiota can, in some cases, contribute microorganisms to different food matrices that will not be cooked. This is why a prior disinfection process must be carried out without this implying that the desired aromatic substances, the favorable flavor, or the functional characteristics of the CSP are affected. Specifically for the cocoa shell, such evaluations have not been carried out. Still, methods used to sterilize cocoa mass can be used, such as applying moisture heat at temperatures below 150°C.
3.6 Cooking properties
Dimensional changes during cooking are mainly attributed to the release of water and fats that decrease their retention in the matrix due to proteins' denaturation. In cooked burgers, no significant difference (p > 0.05) in thickness increase was observed among the treatments (Table 6). Similar values were reported by Pietrasik et al. (2020), although the control properties depend exclusively on the different cuts of meat. On the contrary, diameter reduction and volume loss decreased significantly (p < 0.05) with the addition of fiber but without differences (p > 0.05) between the two treatments. In this context, the preservation of the burger dimension after cooking is of great importance to maintain quality standards; this behavior can be attributed to the stabilization by the effect of the bonds that can be formed between the polar groups of proteins and fibers, decreasing distortion due to the effect of temperature increase (Sánchez-Zapata et al., 2013).
| Cooked burger | Thickness increase (%) | Diameter reduction (%) | Volume loss (%) | Moisture retention (%) | Weight loss (%) | Fat retention (%) |
|---|---|---|---|---|---|---|
| Control | 22.0 ± 6.1 a | 19.3 ± 2.6 a | 21.81 ± 5.1 a | 83.5 ± 0.6 a | 30.97 ± 2.6 a | 52.74 ± 5.9 a |
| CSP1.5% | 24.1 ± 5.0 a | 15.8 ± 2.7 b | 11.50 ± 6.2 b | 85.1 ± 1.4 b | 24.65 ± 1.8 b | 63.98 ± 4.7 b |
| CSP3.0% | 21.4 ± 5.5 a | 16.1 ± 2.0 b | 13.74 ± 6.0 b | 83.7 ± 0.8 a | 25.12 ± 2.0 b | 69.88 ± 2.6 c |
- Notes: Results are expressed as means of four samples ± SD. Different letters in the same column indicate significant differences (p < 0.05) according to Tukey’s multiple-range test.
The moisture retention showed an increase when CSP1.5% was added but not for CSP3.0%, which corroborates the low WHC found, so heating causes moisture not to be retained in a more significant proportion as reported for the addition of hazelnut skin to chicken burgers (Longato et al., 2019). Fat retention presented a statistically significant increase (p < 0.05) concerning the control. However, the OHC was low; this may indicate that the retention was determined by additional interactions in the matrix caused by the CSP. In general, the increase in fat retention can lead to a lower aroma sensation in the burger. Finally, the weight loss that is of great importance to maintain quality standards was lower in the treatments concerning the control due mainly to the lower fat loss, corroborated in the proximal analysis that showed a higher fat content after the cooking in the treatments, although without differences between them (Table 3).
3.7 Volatile compounds
Table 7 shows volatile compounds detected by HS-GCMS; only those compounds showing a significant difference in % of peak areas are shown. In particular, hexanal and 2-butoxyethanol resulted correlated to formulation with CSP; in fact, in Control were detected higher level of hexanal than CSP1.5%, while only very low levels of hexanal were detected in CSP3.0% at the end of the period of observation, suggesting a role of the antioxidant of cocoa shell in preserving cooked meat oxidation during 120 h.
| 0 h (start of storage time) | After 72 h | After 120 h | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Control | CSP1.5% | CSP3.0% | Control | CSP1.5% | CSP3.0% | Control | CSP1.5% | CSP3.0% |
| hexanal | 74.2 ± 2.3aA | 26.02 ± 1.5cB | n.d. | 74.82 ± 2.9aA | 30.92 ± 1.7bB | n.d. | 65.76 ± 5.4bA | 33.17 ± 3.1aB | 2.25 ± 0.9C |
| 2,3-octen-1-ol (Z) | 1.92 ± 0.3b | n.d. | n.d. | 7.63 ± 2.5a | n.d. | n.d. | 8.59 ± 1.7a | n.d. | n.d. |
| 2,3-octanedione | 19.86 ± 2.1a | n.d. | n.d. | 15.02 ± 3.0b | n.d. | n.d. | 9.95 ± 1.6cA | 4.77 ± 2.0aB | n.d. |
| octanal | n.d. | n.d. | n.d. | n.d. | 2.96 ± 0.8a | n.d. | 5.18 ± 1.3aA | 3.44 ± 0.2aB | n.d. |
| nonanal | 6.36 ± 1.0a | n.d. | n.d. | 9.38 ± 0.4a | n.d. | n.d. | 11.53 ± 2.0a | n.d. | n.d. |
| (2-aziridinylethyl)amine | n.d. | 5.99 ± 0.6b | n.d. | n.d. | 4.07 ± 0.4c | n.d. | n.d. | 7.83 ± 1.3aA | 5.78 ± 0.7B |
| ethylbenzene | n.d. | 2.14 ± 0.2b | n.d. | n.d. | n.d. | n.d. | n.d. | 9.61 ± 2.1a | 8.92 ± 2.4a |
| 2-butoxy-ethanol | n.d. | 27.55 ± 3.3a | n.d. | n.d. | 13.46 ± 3.1c | n.d. | n.d. | 16.04 ± 0.8bB | 20.37 ± 2.0A |
- Notes: n.d., not detected. Results are expressed as means of three samples ± SD. Data followed by different letters, in the same line, are significantly different (p < 0.05) according to Tukey’s multiple-range test, lowercase letters indicate the comparison among different storage time for the same sample; capital letters indicate the comparison among different samples at the same storage time.
Hexanal, 3,5-octadien-2-one, 1-pentanol, pentanal are considered products of autoxidation; in particular, hexanal is considered a good marker for measuring oxidation, even if, in this study, its results did not correlate to rancidity detected by sensory analysis (no significant difference).
On the other hand, in burgers with CSP1.5%, we detected 2-butoxyethanol, absent in control samples. This branched-chain alcohol is mainly formed by lipid oxidation, and their amounts increase after the heating process of meat (Park et al., 2009).
3.8 Sensory evaluation
The sensory evaluation with the trained panelists in burger samples immediately after cooking showed characteristic odor and flavor; all panelists attributed score 1 (absent) for off-flavor and off-odor descriptors; scores increased after 72 h of refrigerated storage (4°C) for all descriptors, in accordance to other authors (Rhee et al., 2005). However, most of the descriptors, above those responsible for off-flavor, did not show significant difference among investigated samples, demonstrating no effect of CSP on the change of sensory traits during refrigerated storage of cooked burgers (Figure 2).
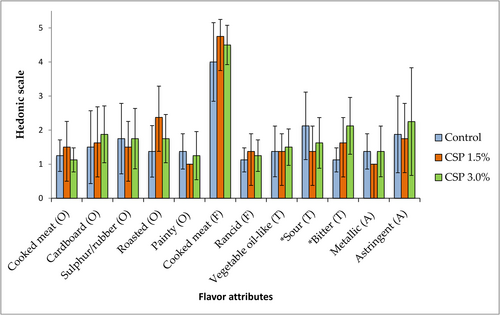
The cooked meat flavor increased with CSP addition. Thermally generated aroma volatiles influence the taste and flavor of cooked meat. Rhee et al. (2005) demonstrated the flavor deterioration in cooked stored meat from all species (pork, beef, chicken). Several factors including peptides, fatty acids, amino acids, vitamins, and fat content play a crucial role in determining sensory attributes (taste and flavor) of the meat; in particular, proteins, lipids, and carbohydrates, when heated, can develop numerous secondary metabolites which are flavor precursors (Ramalingam et al., 2019). Many lipid peroxidation products are volatile compounds such as aldehydes, responsible for off-flavor and off-odor (Campo et al., 2006).
4 CONCLUSIONS
The cocoa shell incorporation into the hamburger formulation represents an important source of PUFA. Our findings suggest that CSP improved the hamburger’s cooking properties. On the other hand, CSP had an essential contribution to shelf life in the evaluated period, probably due to polyphenols’ presence; further studies will be addressed to demonstrate this hypothesis.
The results suggested that burger with the addition of cocoa shell is a new formulation of meat products with high potential to meet consumer demand, being the consumer’s decision to purchase guided by the perception of healthiness and the sensory traits.
5 ACKNOWLEDGMENTS
The authors acknowledge the support provided by Universidad de San Buenaventura Cali, University of Teramo (FARDIB-2019), and Miguel Hernández University. This work was supported by Colciencias, Patrimonio Autónomo Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación Francisco José de Caldas. (C. 808-2018. Agreement 240-2019. Number 123280864259).
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Johannes Delgado-Ospina: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; software; validation; writing – original draft; writing – review and editing. Raquel Lucas-Gonzlez: Methodology. Manuel Viuda-Martos: Conceptualization; methodology; validation; visualization; writing – review and editing. Juana Fernndez-Lpez: Conceptualization; investigation; visualization. Jos Angel Prez-Alvarez: Conceptualization; investigation; resources; supervision; validation; writing – review and editing. Maria Martuscelli: Conceptualization; investigation; methodology; visualization; writing – review and editing. Clemencia Chaves-Lpez: Conceptualization; investigation; supervision; validation; writing – review and editing.
Open Research
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author.




