Virgin olive oil processing by high voltage electrical discharge or high hydrostatic pressure
Funding information: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Abstract
We test the application of two emerging technologies to virgin olive oil (VOO) processing. high voltage electrical discharge (HVED) was tested at 20 kV and about 100 kJ/kg of VOO. High-pressure processing (HPP) was applied for 360 s at about 600 MPa. VOO quality indices were evaluated immediately after treatment and after 6 months. The HPP treatment was found to have no negative influence on any aspect (p > .05 according to General Linear Model and the Tukey HSD test). The HVED treatment did not affect conventional quality indices (p > .05), but was consistent with the appearance of secondary oxidation products (anisidine value increment of about 50% and rDigs increment of roughly 200%, both at p ≤ .05), a significant fall in biophenol concentrations roughly from 5 to 10% at p ≤ .05; and a decrease of about 15% at p ≤ .05 in positive volatiles. Overall, HVED processing appears to negatively impact VOO quality.
Novelty impact statement
- For the first time, high voltage electrical discharge (HVED) has been tested on virgin olive oil (VOO) in comparison to high-pressure processing (HPP) over a storage period
- While HPP was entirely safe for VOO quality, HVED determines a potential detrimental effect
- Our findings support the application of these technologies to food containing VOO, or for its direct treatment, for instance, to protect against microbial spoilage.
1 INTRODUCTION
Consumers demand food products with an ever-better organoleptic profile, and greater health and nutritive properties. At the same time, there is growing awareness of the environmental sustainability of products and processes, and the use of fossil fuels. These issues are encouraging the development of emerging technologies (Knorr et al., 2011; Misra et al., 2017). Sun (2014) notes that although new techniques are all in an early stage of development or implementation at an industrial scale, they have the potential to change how things are done. They can reduce processing time, improve extraction yield (via accelerated mass transfer), preserve sensory properties (via non-thermal processing), and reduce or avoid the use of solvents, saving energy. Examples include electro-technologies (pulsed electric field, high voltage electrical discharge, Ohmic heating, non-thermal plasma), electromagnetic radiation technologies (microwave, radio-frequency drying, pulsed light), and mechanical technologies (high pressure or ultrasound processing) (Knorr et al., 2011; Lasekan et al., 2017; Misra et al., 2017; Sun, 2014). Some of these technologies share the important feature to be non-thermal techniques able to assists the food treatment at low-mild temperature and this aspect could be important for VOO since it is particularly sensitive to temperature during processing (Goulas et al., 2015).
Some of the cited technologies have already been extensively studied or tested on VOO. The topic has been recently reviewed by Pérez et al. (2021).
Among the other, an emerging technology that has already been proposed for use with VOO is high-pressure processing (HPP), although there are only two studies in the literature. Andreou et al. (2017) compare the effectiveness of HPP on olive fruits with pulsed electric field (PEF) processing. Both technologies were found to improve extraction yield and oil quality. Guerrini et al. (2020) treated filtered and un-filtered VOO with HPP. The purpose of the latter study was to gain a deeper insight into VOO stability as a function of the presence of residual water and microbial activity. An interesting result was that HPP is effective in VOO preservation when residual water (i.e., un-filtered VOO) is still present in the oil, due to the strong microbial inactivation caused by the treatment.
However, the application of HPP to VOO needs further examination. Two recent reviews clearly demonstrate that HPP affects the kinetics of lipid oxidation and volatile formation (Medina-Meza et al., 2014; Xia et al., 2020). This is mainly due to HPP-induced changes in the thermodynamic equilibrium of chemical and enzyme-mediated biochemical reactions. Further research into the use of HPP would provide insights into VOO degradation with respect to both the lipid matrix and the volatile profile.
Pérez et al. (2021) report that among the electro-technologies, PEF has been studied in six experiments. All use basically the same approach, namely the treatment of fruit/ olive paste to increase extraction yield, improve process efficiency (by reducing processing time), and produce higher-quality VOO. The only other electro-technology that has been tested on VOO is non-thermal plasma, in the study by Amanpour et al. (2019). In this case, the treatment was directly applied to VOO samples in an attempt to establish the effects on biophenols, aroma compounds, and antioxidant and enzymatic activity. The results highlight that residual enzymatic activity in the oil fell by about 40% with respect to the lipoxygenase (LOX) pathway. At the same time, significant differences were found in biophenolic and volatile profiles.
High voltage electrical discharge (HVED) is another promising emerging technology (Barišić et al., 2022; Boussetta & Vorobiev, 2014; Dalvi-Isfahan et al., 2016; Li et al., 2019). In this case, it has never been tested on VOO. The technique consists of the application, typically in a liquid medium, of short, high voltage electrical discharges. The most common configuration is a point-plane electrode system that is submerged in water, typically consisting of two consecutive steps (streamer and breakdown) depending on the increase in the applied voltage. The underlying mechanism creates a plasma region around the electrode, via ionization of the liquid being treated. High-intensity UV emissions are followed by a shock wave and the formation of radicals (hydroxyl, if water is the medium). In theory, HVED can also be applied to vegetable oils, although they are weakly conductive. VOO has been found to have specific electrical properties. Resistance is of the order of 1.00–3.00E+9Ωm and breakdown voltage is about 80 [email protected] mm (Reffas et al., 2018). Reffas et al. (2018) also report that impulsive high voltage discharges (either positive or negative) in VOO result in streamer formation with a potential transition to complete breakdown as a function of the applied voltage.
The present work aims to add to the body of knowledge regarding the application of emerging technologies to VOO processing. This could lead to, eventually, either a direct VOO treatment (e.g., microbial inactivation that would avoid the need for filtration), or the ability to predict the response of food preparations in which VOO is a part of the formula (Kumar, 2015; Moretto et al., 2020; Nieto & Lorenzo, 2021; Özer & Çelegen, 2021; Punia et al., 2020). At this purpose, we compare the direct application of HVED and HPP to VOO samples. We investigate the qualitative effects in detail, notably any potential changes in fatty acid composition, oxidative indices, and biophenolic and volatile profiles, both immediately after treatment and after 6 months of storage. This is the first step in understanding the feasibility of the application of HVED to VOO processing, while for HPP we provide a better understanding of the qualitative consequences of the application of the technique.
2 MATERIALS AND METHODS
2.1 Olive oil
About 10 kg of freshly produced VOO was procured directly from the mill and processed within 7 days of production. The mill was equipped with a continuous extraction plant (Cultivar 750/3GV_400, MORI-TEM srl, Italy). A mixed batch of olive fruit cultivars Frantoio and Moraiolo (proportions unknown) was processed.
2.2 High-pressure processing
VOO samples were placed in 250 ml PET bottles for HPP treatment. The equipment was made available for testing by HPP Italia srl (Traversetolo, Parma, Italy) and consisted of a JBT Avure™ HPP industrial plant (AV-40X, Avure Technologies, USA). The main technical features are as follows: a high-pressure pump; a 525 L standard treatment vessel, with an internal diameter 0.471 m and internal length 3.000 m; cooling water flow rate 300 L min−1 at 1.0°C; and 725 kW power supply (850 kVA, 3-phase, 400 V, 50 Hz).
Two series (three bottles per series) of samples were treated in order to be able to characterize the VOO immediately after treatment, and after 6 months of storage. Once in the standard treatment vessel, pressure was increased at an average rate of 3 MPa s−1, until the final pressure of 608 MPa was reached. Treatment time was set at 360 s, with continuous cooling (using water at about 19 ± 1°C) to prevent the oil heating during compression, or freezing during the quasi-instantaneous drop of pressure at the end of the treatment. The first series of treated samples were stored at 5 ± 1°C in dark conditions, before chemical analyses, which were run within 1 week. The second series of treated samples were stored for the shelf-life test.
2.3 High voltage electrical discharge
We developed a bespoke HVED experimental apparatus. The treatment chamber consisted of a cylindrical cell with an internal diameter of 148 mm, height of 200 mm, and total volume of 3439 ml. We adopted the conventional configuration of point-plane electrodes. The plane electrode was fixed to the inner base of the treatment chamber, which was made of a 3 mm steel disk (inox AISI 316), and supported and insulated by a 15-mm-thick Teflon disk. The wall of the chamber consisted of an acrylic tube (inner diameter 148 mm, wall thickness 5 mm). The point electrode was made of a steel rod with a diameter of 6 mm, length of 300 mm, and a conical end with an aperture angle of 31.33°. Once filled with VOO (250 g per test), the point electrode was immersed in the oil to a height of about 6 mm, at a fixed distance (10 mm) from the plane electrode (the treatment gap). Electrical discharge used a high voltage zero-volt-switching driver board fitted to a fly-back transformer (BSC2401N4014K, Ometter Electronic Ltd, China). The driver board was connected to a dedicated 24 V, 10 A, 240 W direct current power supply.
2.4 Experimental procedure
The experiment was designed to be analyzed with a conventional general linear model (GLM). Two main sources of variation (factors) and their interaction were tested in triplicate. The first factor was the innovative treatment, studied at three levels (i.e., HPP, HVED, and the control). The second was storage time, studied at two levels (immediately post-treatment, and 6 months after treatment).
Immediately after collection at the mill, the VOO batch was taken to the lab and conditioned at 20°C for 60 min under slow stirring. Then, it was divided into three aliquots: one for the HPP treatment; one for the HVED treatment; and one to be used as a control. The HPP aliquot was bottled in six PET bottles (250 ml nominal volume) and sent to the treatment plant. With respect to the HVED treatment, 250 g VOO samples sequentially underwent treatment until six bottles were obtained (three for immediate analysis, three for 6-month storage). The control aliquot was bottled (three for immediate analysis, three for 6-month storage). Bottles were stored in a dedicated chamber (1.3 m wide, 0.8 m long, 1.0 m deep) with a reflective material (tin foil with a thickness of about 0.2 mm) covering the inner walls. They were randomly positioned inside the chamber, and equally spaced according to a pre-established grid. Both temperature (20 ± 1°C) and illumination were controlled. Illumination (Master TL-D 90 Graphica lamp, 35 W/390, Philips, Amsterdam, The Netherlands) was held at 1900 LUX, alternating dark (12 hr) and light (12 hr) cycles.
2.5 Chemical analyses
Free acidity, peroxide value, UV-specific extinction coefficients, and fatty acid composition were determined following the EEC specification (EC, 2008). Total and individual biophenols were assessed according to the method given by International Olive Council (COI/T.20/Doc No 29., 2009).
Head space/ solid phase microextraction/ gas chromatography/ mass spectrometry (HS-SPME-GC–MS) was used to identify and quantify VOO volatiles following the method given in Masella et al. (2019). This identified 52 compounds, grouped as follows: ∑C5 (the sum of compounds with five carbon atoms belonging to the LOX pathway) = 2-Pentenal, 1-Penten-3-ol, E-2-penten-1-ol, Z-2-penten-1-ol; ∑C6 (the sum of compounds with six carbon atoms belonging to the LOX pathway) = Hexanal, Z-3-Hexenal, E-2-hexenal, Acetic acid hexyl ester, E-2-hexenyl acetate, E-3-hexen-1-ol, Z-3-hexenyl acetate, Z-3-Hexen-1-ol, Z-2-hexen-1-ol, E-2-Hexen-1-ol, 1-hexanol; ALD (aldehydes) = Heptanal, E-2-heptenal, Nonanal, 2,4-Hexadienal, E-2-Octenal, 2,4-Heptadienal, Decanal, Benzaldehyde, E-2-Decenal, 2,4-Nonadienal, 2,4-Decadienal; EST (esters) = methyl acetate; KET (ketones) = Butan-2-one, Pentan-2-one, 1-Penten-3-one, Heptan-2-one, 1-octen-3- one, Nonan-2-one; AC (organic acids) = acetic acid, propanoic acid, butanoic acid, pentanoic acid, hexanoic acid; ALC (alcohol) = Methanol, Butan-2-ol, Propanol, 3-Methyl-1-butanol, Heptan-2-ol, Octan-2-ol, 1-octen-3-ol, Heptan-1-ol, Octan-1-ol, Nonanol, Phenylethyl Alcohol; and PHEN (phenols) = Guaiacol, 4-Ethylphenol, 4-Ethylguaiacol.
The ratio of 1,3 to 1,2 diacylglycerol isomers (rDigs) was determined according to the method given in Pérez-Camino et al. (2001). Anisidine value (AV) was determined according to the method given in ISO 6885 (2006).
2.6 Statistical analysis
A general linear model (GLM) was applied and analyzed by conventional multi-way ANOVA at a significance level ranging from 1% (p ≤ .01) to 10%(p ≤ .10). Where the ANOVA was significant, a post hoc Tukey LSD test was applied, with the same significance levels used in the ANOVA.
3 RESULTS AND DISCUSSION
The experimental VOO batch was immediately sampled before treatment and checked in triplicate for possible microbial contamination. Other checks were made as the experiment was run. As no sign of contamination was found, we can exclude the effect of residual microorganism metabolism activity on VOO quality.
VOO quality can be determined in various ways. The most basic requirement is regulatory standards. Free acidity, peroxide value, and UV extinction coefficients determine the commercial category of VOO, and reflect its hydrolytic (free acidity) and oxidative state. The first question that must be answered when a new treatment is tested is whether it has a negative effect on these quality indices. Thus, Table 1 reports the mean values of key parameters revealed by the GLM. This table shows that only the main effect of storage time was significant (p ≤ .01) for peroxide value and UV extinction coefficients; this is expected as storage time is consistent with oxidative deterioration. Neither the HPP nor the HVED treatment had a negative effect on oxidative indices.
| Free acidity (%) | Peroxide value (meq.O2 kg−1) | K232 | K268 | |
|---|---|---|---|---|
| Time (months) main effect | ||||
| 0 | ns | 6.62 (0.96)b | 1.65 (0.02)b | 0.13 (0.01)b |
| 6 | ns | 9.46 (1.84)a | 1.99 (0.02)a | 0.17 (0.01)a |
| Treatment main effect | ||||
| control | 0.25 (0.01)b | ns | ns | ns |
| HVED | 0.27 (0.01)a | ns | ns | ns |
| HPP | 0.24 (0.01)b | ns | ns | ns |
- Note: Values indicated by letters are significant at p ≤ .05 (GLM analysis and the Tukey HSD test).
- Abbreviations: HVED, high voltage electrical discharge; HPP, high-pressure processing; ns, not significant.
Referring to the HPP treatment, this result is fully consistent with data presented by Guerrini et al. (2020), where no significant increment of conventional oxidative indices occurred after 6 months of olive oil storage. Data about the oxidative effect of HVED, to the best of our knowledge, are lacking in literature. The closest technology is probably the non-thermal plasma proved on VOO by Amanpour et al. (2019). Such author find a non-significant increment of peroxides in treated VOO samples, with a variation smaller than 2%.
Interestingly, in our case, a significant difference was found for free acidity. Specifically, values were very slightly higher for samples treated with HVED (about 8% compared to the control). Further insight into the potential oxidative effects of the two treatments was gained from the AV and the rDigs value. Results are summarized in Figure 1. The GLM identified significant interactions for both parameters. For the AV, the effects of the HVED and HPP treatments were not significantly different to the control at time 0, but the value increased significantly during storage in the HVED condition compared to the HPP condition. A similar result was found for rDigs, where values were markedly higher in the HVED condition after 6 months of storage compared to both the HPP treatment and the control.
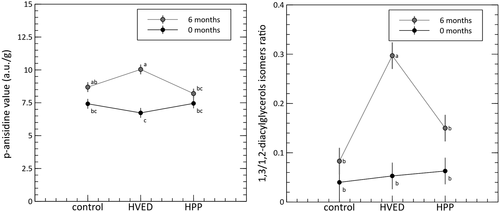
Few recent data about AV values of VOO are present literature, none referring to HPP or HVED treatments. Cobzaru et al. (2016) give AV values higher than our data in a shelf-life test, starting from about 15 a.u./g AV at time 0 of storage, to about 40 a.u./g AV at 12 months of storage. Values reported by Cerretani et al. (2009) are closer to our data, spanning around 5 a.u./g AV. Interestingly, in such paper, the oxidative stress induced by 3 minutes microwave treatment leads to a AV increase up to about 10 a.u./g, that is, a value close to the oil samples undergoing the HVED treatment after 6 months of storage. As for AV, data about the rDigs parameter as affected by HPP or HVED, are lacking. However, our data are fully comparable to other reports about VOO (Pérez-Camino et al., 2001).
The above results are supported by the analysis of the fatty acid composition of the treated samples, where several significant effects were found. Seventeen fatty acids were identified. In 11 cases, the GLM was significant and, more specifically, the interaction was significant in seven of these 11 cases. To clarify the interpretation and presentation of our results, fatty acids were grouped into three classes based on their degree of saturation: saturated fatty acids (SFA); monounsaturated fatty acids (MUFA); and polyunsaturated fatty acids (PUFA), and the GLM was recomputed on these grouped data. Following this grouping, the interaction between treatment and time was significant for SFA (p ≤ .10) and MUFA (p ≤ .05), while for PUFA the main effect of storage time dominated (p ≤ .05) (Figure 2). Like free acidity, these differences in the fatty acid profile were limited, but nevertheless significant and could indicate a breakdown in the carbon chain as a response to the HVED treatment.
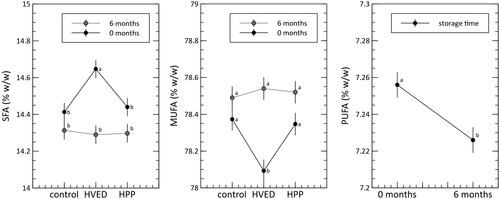
In both of the tested conditions, it was reasonable to expect oxidative degradation of the lipid matrix. This observation is supported by the literature, where two recent reviews have examined lipid oxidation as a response to HPP (Medina-Meza et al., 2014) or HVED treatments (Gavahian et al., 2018). In the case of HPP, the main theoretical reason to expect lipid degradation relates to modification of the thermodynamic equilibrium of chemical reactions. Specifically, oxidation reaction rates are expected to change with the application of intense pressure. Moreover, the extent of such an effect is expected to be a function of the applied pressure, with indicative thresholds of about 300–500 MPa. In our experiment, none of the considered oxidative indices were worsened by the HPP treatment either immediately after processing, or after storage. We conclude that the VOO fatty matrix appears to be resilient to the application of HPP. This was not the case, however, for the HVED treatment, which seems to have a detrimental effect. Although this was not evident from the standard oxidative indices, it was revealed by the AV and rDigs. The former relates to the presence of secondary products (mainly aldehydes, conjugated dienals and 2-alkenals) from unsaturated fatty acid oxidation. The rDigs value reflects a shift in the proportion of 1,2 to 1,3-diacylglycerols derived from isomerization, which is generally a function of storage (time and temperature) and the extent of lipolysis (i.e., the level of free acidity). In our experiment, the HVED treatment affected the oil's fatty acid profile, increased the level of free acidity, increased the AV, and increased the ratio of 1,3-diacylglycerols. All of these changes are consistent with oxidative/ hydrolytic stress induced by the electrical discharge in the treated VOO. Gavahian et al. (2018) argue that these effects may be due to the formation of reactive oxygen species that are able to initiate lipid oxidation. This is especially true when HVED is used in an un-submerged configuration under gas flow in water (Bruggeman & Leys, 2009). Although the present study used a submerged configuration without external gas flow, the tested VOO samples contained a residual amount of water (roughly 0.2% by weight). It is also probable that the samples contained a certain amount of dissolved oxygen. While we did not measure it in this experiment, Parenti et al. (2007) found that freshly produced VOO generally has a dissolved oxygen content of about 5–9 mg L−1. Thus, it is likely that the applied electrical discharge led to the formation of hydroxyl radical, hydrogen peroxide, ozonide, etc., along with strong UV radiation. All of which contribute to explaining the observed oxidative effect (Režek Jambrak et al., 2021).
VOO quality can also be determined by the amount and profile of two classes of minor components, namely biophenols and volatiles. For the former, the first key indicator is total content, evaluated using the International Olive Council method (COI/T.20/Doc No 29., 2009). The GLM identified significant main effects for both of the tested factors (Figure 3). As expected, storage time was consistent with a fall in biophenolic content, independent of the treatment, and no interaction was found. However, concentrations were significantly higher in the HPP condition. The effects of HPP on the phenolic matrix cannot be predicted on the basis of the current literature (Khan et al., 2018) and potential effects appear to be a function of treatment conditions such as temperature and time, but, most importantly, pressure.
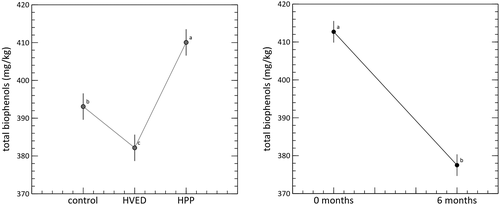
Guerrini et al. (2020) did not found a significant effect of the HPP treatment of VOO, under treatment conditions similar to those of the present study.
The food matrix is, of course, a relevant factor in comparisons with alternative high-temperature treatments. In general, it seems that phenols are resilient to HPP, and that high-pressure treatments may indirectly affect their amount by enzymatic deactivation in the matrix. It is generally accepted that biophenol kinetics in VOO rely either on hydrolytic or oxidative reactions (Zanoni, 2014). The latter, in turn, can be affected by the oil's residual water content, and the residual activity of enzymes such as polyphenol oxidase. In the present experiment, it is conceivable that the early application of the HPP treatment (a few hours after production) determined a sharp slowdown in degradation reactions, which occurred normally in the control and HVED VOO samples. This also implies that the HVED treatment did not have the same preservation effect.
The significant increase in biophenol concentration that was found after the HPP treatment was maintained during storage (Figure 3). Figure 3 also shows that concentrations were significantly lower in VOO treated with HVED. The absence of an interaction between treatment condition and time suggests that total biophenols were not affected by the initial treatment. At time zero, the HVED treatment was consistent with a significant reduction in biophenolic content, and this reduction was maintained during storage. This result confirms the observation of Amanpour et al. (2019), which found a significant decrease of total biophenol of about 16% as a consequence of non-thermal plasma application.
However, this behavior was not consistent for every compound. In some cases, there was a main effect of storage, for others the interaction was significant, and for other individual compounds no main effect of treatment was detected. Table 2 shows that there was a main effect of storage time for 13 biophenols. In eight cases, this corresponded to an increment, which may be ascribed to the degradation of complex biophenols into simpler compounds (e.g., hydroxytyrosol and tyrosol derived from decarboxymethyl oleuropein and ligstroside aglycones, respectively). However, overall these compounds only accounted for about 10% of total phenolic content. In contrast, concentrations of the remaining five compounds fell (aldehyde and hydroxylic forms of ligstroside aglycone; decarboxymethyl oleuropein aglycone dialdehyde; and oxidized aldehyde and hydroxylic forms of ligstroside aglycone), which were most representative in quantitative terms.
| Specific biophenols | 0 months | 6 months |
|---|---|---|
| Hydroxytyrosol | 1.5 (0.21)b | 4.55 (0.42)a |
| Tyrosol | 1.88 (0.11)b | 2.79 (0.12)a |
| Vanillic acid + Caffeic acid | 1.74 (0.21)a | 1.13 (0.13)b |
| Vanillin | 0.62 (0.15)b | 2.63 (0.75)a |
| Para-coumaric acid | 2.1 (0.34)a | 0.97 (0.54)b |
| Hydroxytyrosyl acetate | 0.75 (0.13)b | 5.39 (1.94)a |
| Decarboxymethyl oleuropein aglycone, dialdehyde | 121.17 (5.04)a | 103.93 (6.52)b |
| Oleuropein | 7.05 (2.14)b | 16.37 (3.83)a |
| Oleuropein aglycone, dialdehyde | 1.98 (0.32)b | 6.22 (3.56)a |
| Cinnamic acid | 1.47 (0.24)b | 2.15 (0.52)a |
| Ligstroside aglycone, oxidized aldehyde, and hydroxylic | 30.73 (2.43)a | 25.47 (2.4)b |
| Methyl-luteolin | 4.47 (0.26)b | 5.04 (0.6)a |
| Ligstroside aglycone, aldehyde, and hydroxylic | 51.07 (1.95)a | 45.36 (1.6)b |
- Note: Values indicated by letters are significant at p ≤ .05 (GLM analysis and the Tukey HSD test).
Table 3 shows that some phenols behaved differently during storage as a function of the initial treatment, notably decarboxymethyl ligstroside aglycone dialdehyde. This compound tended to decrease over time in control samples, but significantly increased in both treated samples. Although concentrations of all other compounds fell significantly over time, there were no significant differences at the end of storage. The exception was the oxidized form of decarboxymethyl ligstroside aglycone dialdehyde, which increased significantly in both treated and control samples, but with a higher increase in the HPP treatment.
| 0 months | 6 months | |||||
|---|---|---|---|---|---|---|
| Control | HPP | HVED | Control | HPP | HVED | |
| Ferulic acid | 3.87 (0.38)b | 5.5 (0.88)a | 4.05 (0.49)b | 0.71 (0.14)c | 0.93 (0.39)c | 0.93 (0.2)c |
| Decarboxymethyl oleuropein aglycone, oxidized dialdehyde | 31.19 (3.07)a | 30.79 (1.86)a | 25.48 (3.14)a | 8.5 (1.13)b | 7.35 (3.85)b | 12.27 (4.87)b |
| Decarboxymethyl ligstroside aglycone, oxidized dialdehyde | 10.19 (0.74)c | 9.35 (0.17)c | 8.94 (1.02)c | 12.43 (2.2)bc | 17.51 (2.36)a | 14.84 (0.23)ab |
| Decarboxymethyl ligstroside aglycone, dialdehyde | 35.63 (0.62)bc | 35.9 (0.16)b | 32.51 (1.1)d | 33.46 (0.48)cd | 38.29 (1.26)a | 34.97 (0.94)bc |
| Pinoresinol, 1 acetoxy-pinoresinol | 40.57 (0.32)bc | 49.42 (5.43)a | 42.07 (1.37)b | 40.19 (0.33)bc | 40.53 (0.92)bc | 35.13 (2.39)c |
| Oleuropein aglycone, aldehyde, and hydroxylic | 45.35 (1.7)a | 40.39 (4.09)ab | 43 (4.57)ab | 32.02 (1.06)c | 35.49 (1.62)bc | 30.1 (1.34)c |
- Note: Values indicated by letters are significant at p ≤ .05 (GLM analysis and the Tukey HSD test).
- Abbreviations: HVED, high voltage electrical discharge; HPP, high-pressure processing; ns, not significant.
The final qualitative aspect relates to the volatile fraction. VOO aroma is due to the olfactory characteristics of a set of volatile organic compounds (esters, aldehydes, ketones, alcohols, etc.) that derive from multiple biosynthetic pathways (Angerosa et al., 2004). The most important, especially in high-quality oils, is the LOX pathway, which leads to the formation of volatiles with six (C6) and five (C5) carbon atoms. The latter encompass esters, alcohols, aldehydes, and ketones that are produced enzymatically from polyunsaturated fatty acids (linoleic and linolenic acid). The second nodal pathway for volatile formation is lipid matrix autoxidation (Choe & Min, 2006). In this case, unsaturated aldehydes dominate.
The present work quantified up to 52 compounds. For clarity, they are grouped into C5 and C6 compounds from the LOX pathway on the one hand, and by chemical family on the other (aldehydes, ketones, alcohols, acids, esters, and phenols). The GLM was applied to these groups, and the results are summarized in Table 4. The first important finding relates to C6 compounds. Here, E-2-hexenal initially dominates, but falls significantly in response to HVED processing. In contrast, the C5 group was not affected by the treatment. The second important finding is a significant increase in aldehydes, both as a function of storage time or the HVED treatment. These two results indicate that the HVED process has a detrimental effect, both in terms of decreased concentrations of compounds that are generally linked to a positive aroma, and in terms of an increased concentration of unsaturated aldehydes, which is closely linked to lipid matrix oxidation. The latter result was also found for storage time, due to the progressive oxidation of the lipid matrix.
| Volatiles group# (μg kg−1) | 0 months | 6 months | ||||
|---|---|---|---|---|---|---|
| Control | HPP | HVED | Control | HPP | HVED | |
| ∑C5 compounds | 929 (33)ns | 883 (66)ns | 864 (66)ns | 927 (26)ns | 903 (22)ns | 882 (64)ns |
| ∑C6 compounds | 43,262 (2573)a | 41,323 (1838)a | 35,049 (5127)b | 40,190 (548)a | 40,332 (2320)a | 38,237 (469)b |
| Aldehydes | 3337 (233)b,x | 3749 (158)ab,x | 3682 (242)a,x | 4338 (510)b,y | 4241 (575)ab,y | 5268 (351)a,y |
| Alcohols | 11,792 (3668) | 2509 (295) | 5617 (248) | 11,132 (3693) | 2753 (166) | 2866 (312) |
| Esters | 36 (19)x | 55 (13)x | 45 (20)x | 71 (20)y | 72 (18)y | 69 (23)y |
| Acids | 641 (273)ns | 496 (92)ns | 710 (256)ns | 359 (61)ns | 433 (227)ns | 587 (103)ns |
| Ketones | 10,619 (2948)ns | 13,387 (787)ns | 12,396 (1534)ns | 13,618 (1489)ns | 13,294 (2164)ns | 12,959 (2408)ns |
| Phenols | 517 (4)ns | 462 (25)ns | 448 (25)ns | 512 (78)ns | 497 (68)ns | 465 (61)ns |
- # Group composition is given in Section 2.4. Values refer to the mean (standard deviation) of three independent replicates. Letters indicate significance according to the GLM ANOVA and Tukey's HSD test. Letters “a” to “c” (if present) indicate a significant main effect of treatment or an interaction. Letters “x” to “y” (if present) indicate a main effect of storage time. ns = not significant; ∑C5 or ∑C6 = sum of compounds with five or six carbon atoms, belonging to the LOX pathway, as detailed in Section X. HVED = high voltage electrical discharge; HPP = high-pressure processing.
Once again, these results agree with the non-thermal plasma treatment reported by Amanpour et al. (2019), where, for instance, Pentanal and (E)-2-Pentenal were not significantly affected, whereas (E)-2-Hexenal dropped of about 13% and Nonanal undergone a three-fold increase.
A further interesting result that is difficult to understand was a significant fall in alcohols (other than those that are part of the LOX pathway) in both treatment conditions compared to the control. This may simply be due to advanced oxidation following treatment. This would be consistent with the demonstrated oxidative effect of HVED, but not with the HPP treatment where no noticeable oxidation was identified. Moreover, the effect of storage time (clearly an oxidative agent), was not significant. Hence, this aspect needs further exploration.
4 CONCLUSIONS
We investigated the effect of two emerging technologies on VOO quality. Our experiment tested HVED technology for the first time, in moderately intense conditions (20 kV and about 100 kJ/kg specific energy). HPP was applied at high intensity (600 MPa). Both technologies may damage VOO through oxidation of the lipid matrix and/ or degradation of biophenolic and volatile profiles. We report two main findings. First, VOO can be safely treated with HPP. Although the literature underlines a general risk of lipid oxidation, we found no indication of this, or biophenol degradation. Hence, we conclude that the application of HPP does not impair VOO quality. This is also a useful finding regarding the application of HPP to food that contains VOO as a lipid source, notably meat products (Gaforio et al., 2018).
The same finding does not apply to HVED technology, particularly when the effects of the treatment are investigated in depth. Although the main VOO quality indices were not significantly affected, and the oil can still be commercially classified as extra virgin, a deeper analysis revealed clear signs of degradation. In particular, there is a significant change in the oxidative and hydrolytic profile of the oil. Rancidity markers, such as conjugated dienals and 2-alkenals appear, and there is a parallel decrease in positive volatiles that are typically part of the LOX pathway. However, given that this technology may be used to treat food that contains VOO (as a lipid source), the extent of these changes should be assessed more broadly, in terms of the trade-off between its negative effects on the VOO fraction and its positive effects on the treated food, notably microbial inactivation.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Firenze within the CRUI-CARE Agreement. [Correction added on May 9, 2022, after first online publication: CRUI-CARE Funding statement has been added].
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
AUTHOR CONTRIBUTIONS
Giulia Angeloni: Conceptualization; writing – original draft. Lorenzo Guerrini: Data curation. Carlotta Breschi: Investigation. Bruno Zanoni: Formal analysis. Luca Calamai: Data curation. Alessandro Parenti: Data curation; funding acquisition. Piernicola Masella: Conceptualization; data curation; funding acquisition; investigation; methodology; project administration; resources; writing – original draft; writing – review and editing.
Open Research
DATA AVAILABILITY STATEMENT
Author elects to not share data




