Evaluation of Diagnostic Accuracy of the Paris System (TPS 2.0) in Urine Cytology Specimens: An Institutional Experience From a Large Cohort of a Tertiary Care Centre
Funding: The authors received no specific funding for this work.
Hemlata Jangir and Anubhav Narwal are co-first authors.
ABSTRACT
Objective
The objective of this study is to evaluate the diagnostic performance of urine cytology using The Paris System (TPS 2.0) in comparison with TPS 1.0, and the Four-Tier Reporting System (FTRS) of our institute for identifying high-grade urothelial carcinoma (HGUC).
Methodology
A total of 789 urine cytology specimens from 240 patients including 12 UTUC cases with available histological and clinical details were included. Two hundred twenty-two cases were newly diagnosed, whereas 18 were recurrent tumours. Histopathological evaluation categorised the cases as non-neoplastic (33%, 13.7%), low-grade urothelial neoplasms (LGUNs) (94%, 39.2%), high-grade urothelial carcinoma (HGUCs) (110%, 45.8%) and other malignancies (3%, 1.3%).
Results
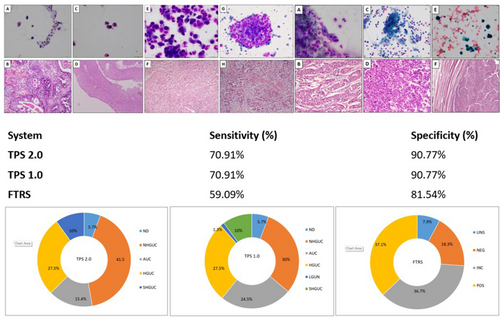
TPS 2.0 categorised the cases as 14 (5.7%) non-diagnostic or ND/U, 99 (41.5%) NHGUC, 37 (15.4%) AUC, 24 (10%) SHGUC and 66 (27.5%) HGUC. TPS 1.0 had 14 (5.7%) ND/U, 72 (30%) NHGUC, 61 (24.5%) AUC, 3 (1.3%) LGUC, 24 (10%) SHGUC and 66 (27.5%) HGUC. FTRS classified them as 19 (7.5%) ND/UNS, 44 (18.3%) NEG, 88 (36.7%) INC and 89 (37.1%) POS. The ROHM for TPS 2.0 was 71.4% for ND/U, 12.1% for NHGUC, 29.7% for AUC, 79.2% for SHGUC and 89.4% for HGUC. TPS 1.0 showed a similar ROHM for ND/U, SHGUC and HGUC, whereas had 13.8% for NHGUC and 19.7% for AUCs. FTRS, had 78.9% for UNS, 6.8% for NEG, 35.2% for INC and 73.1% for POS. TPS demonstrated a sensitivity of 70.91% and specificity of 90.77% for identifying HGUC, whereas FTRS showed 59.09% sensitivity and 81.54% specificity. Also, TPS was found to be 75% accurate with 62.5% sensitivity and 100% specificity for UTCC cases separately.
Conclusion
TPS 2.0 exhibits diagnostic accuracy with better performance in comparison to FTRS, making it a more reliable system for clinical practice. Our findings endorse the utility of TPS 2.0 in improving the accuracy of urine cytology in predicting histological diagnosis of HGUC.
Graphical Abstract
Urine cytology diagnostic performance using TPS 2.0, TPS 1.0 and FTRS for high-grade urothelial carcinoma (HGUC). Our study highlights that the Paris System (TPS) 2.0 significantly enhances the diagnostic accuracy and consistency of urine cytology in detecting high-grade urothelial carcinoma (HGUC), including upper tract urothelial carcinoma (UTUC). The integration of TPS 2.0 with emerging AI and molecular testing can further refine diagnostic precision and clinical management across urothelial neoplasms.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available with the corresponding author.