Acute Restraint Stress Induces Long-Lasting Synaptic Enhancement by Inhibiting AMPK Activation in AD Model Mice
Funding: This study was supported by the Natural Science Foundation of Zhejiang Province (grant number: LQ24H310001 to M.W.); the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: NRF-2021R1I1A1A01050493 to M.W.); the Korea Dementia Research Project through the Korea Dementia Research Center (KDRC), funded by the Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea (grant number: 10.13039/100020206 to J.J.); the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number:10.13039/501100003725 to J.J.); KBRI basic research program through Korea Brain Research Institute funded by Ministry of Science and ICT (grant number: 24-BR-03-05 to J.J.); The Electronics and Telecommunications Research Institute (ETRI) grant funded by the Korean government (MSIP) (grant number: 23YR1600 to J.J.).
Ming Wang and Baoyuan Jin contributed equally to this work.
ABSTRACT
Background
Alzheimer's disease (AD) is characterized by a gradual synaptic loss. The progression of AD severely affects late-phase long-term potentiation (L-LTP), which is essential for long-term memory consolidation.
Aim
We have previously demonstrated the beneficial effects of acute restraint stress (ARS) on hippocampal LTP in AD mouse models. This study aimed to verify the effects and potential mechanisms of ARS on the maintenance of hippocampal L-LTP in two AD mouse models.
Materials and Methods
5xFAD and Tg2576 mice underwent a 30-min body immobilization protocol to induce ARS, followed by electrophysiological recordings of L-LTP (> 3 h) in the CA1 region of thehippocampus.
Results
The ARS-exposed group exhibited significantly enhanced L-LTP compared to the control group. Maintenance of L-LTP requires new protein synthesis and signaling via the mammalian target of rapamycin (mTOR) pathway. Our findings revealed that ARS increased hippocampal adenosine triphosphate (ATP) production and reduced AMPK activity. Inactivation of AMPK and subsequent activation of the mTOR pathway were strongly associated with the ARS-facilitated enhancement of L-LTP. Furthermore, our experiments using the mTOR inhibitor rapamycin demonstrated that it effectively prevented the enhancement of L-LTP following ARS, underscoring the pivotal role of mTOR in this process.
Conclusion
ARS may significantly modify AMPK activation and mTOR regulation in L-LTP, potentially triggering the mechanisms of long-term memory consolidation in AD mouse model mice. Identifying these underlying mechanisms could help promote the development of novel pharmaceutical agents for the treatment of AD.
1 Introduction
LTP represents an increase in synaptic transmission that underlies the cellular process of memory formation [1]. Early-phase LTP (E-LTP) induction is rapid, and its expression does not require new protein synthesis, which is sustained 1 h after the delivery of high-frequency stimulation (HFS). However, a persistent form of synaptic plasticity, L-LTP, requires new protein synthesis, which occurs 2–3 h after an HFS protocol [1-4]. The mammalian target of rapamycin (mTOR) kinase complex modulates protein translation through the phosphorylation of p70S6 kinase (p70S6K) and then subsequently phosphorylates the ribosomal protein S6 (rpS6) [5-7]. mTOR, a key modulator of L-LTP, regulates the translational capacity by promoting protein synthesis [8-10]. Furthermore, rapamycin, a specific mTOR inhibitor, suppresses sustained hippocampal LTP [11]. Moreover, the knockout of upstream mTOR molecules leads to synaptic plasticity deficits and memory loss [12]. However, the potential role of upstream mTOR molecules in L-LTP maintenance remains unclear.
Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a central mediator of energy homeostasis in the central nervous system (CNS) [13-16]. Generally, a reduction in cellular ATP levels regulates AMP, which then activates AMPK via phosphorylation at threonine 172 (Thr172), resulting in accelerated ATP production and reduced ATP consumption [17-19]. Acute stress can increase the release of ATP in the hippocampus [20, 21], indicating a potential effect of acute stress on AMPK activity. In the brain, AMPK hyper-activation inhibits mTOR, resulting in impaired axonal growth [22]. The inhibition of AMPK activity directly enhances synaptic plasticity by mediating mTOR [23]. mTOR signaling is involved in several processes that regulate neurodevelopment and neuronal plasticity. Thus, dysregulation of mTOR signaling has been linked to several brain disorders associated with neuronal dysfunction, such as neurodegeneration and neurobehavioral alterations [24]. The AMPK activators significantly prevent the L-LTP expression, whereas L-LTP maintenance increases with AMPK inhibitors [25]. Furthermore, rapamycin completely diminishes the effect of AMPK inhibitors on L-LTP enhancement [9]. This strongly suggests that the AMPK–mTOR pathway is involved in a protein synthesis dependent on LTP.
AD is an irreversible and progressive neurodegenerative disease that is associated with deficits in cognitive function and memory loss. The “amyloid cascade hypothesis” has provided the main theoretical construct for AD [26, 27]. The best understood AD pathogenesis in CNS is attributed to a loss of plasticity, representing a hippocampal LTP deficit [28, 29].
Stress affects how significant experiences are interpreted and serves as a benchmark for future events through memory [30]. The hippocampus is a target of stress events that cause atrophy of dendrites, and chronic stress events suppress the neurogenesis of hippocampal neurons [31]. However, it has been recently reported that both acute stress and stress hormones can enhance hippocampal LTP through dynamic molecular alterations under normal and abnormal conditions [32-35]. Furthermore, we have previously demonstrated the effect of an ARS protocol on LTP facilitation in the hippocampus of AD model mice, which was mediated by glucocorticoids [36]. This indicates a potential role for acute stress events in impaired synaptic plasticity. However, further clarification is needed regarding the cellular and molecular mechanisms mediating the long-lasting effects of ARS on hippocampal LTP enhancement in AD mouse models.
To address this question, we utilized the transgenic AD mouse models—5xFAD mice and Tg2576 mice. Field recording of hippocampal LTP was performed after body immobilization for 30 mins, and the molecular alterations were examined. We found that the effective duration of ARS facilitated LTP up to 3 h after the tetanus protocol, which is similar to our previous study. Importantly, L-LTP induced by ARS within the AD mice hippocampus was accompanied by a strong inhibition of AMPK activity and an increase in mTOR downstream molecular expressions. In addition, a specific mTOR inhibitor fully abolished L-LTP enhancement using the ARS protocol. These results establish a mechanism for AMPK-mTOR signaling-controlled protein synthesis in the acute stress-facilitated L-LTP. Owing to its potential role in learning and memory, the ability to restore the LTP deficit under pathological conditions may be beneficial for therapy.
2 Material and Methods
2.1 Animals
Male 5-month-old 5xFAD transgenic mice (APP KM670/671NL, APP I716V, APP V717I, PSEN1 M146L, PSEN1 L286V) were obtained from The Jackson Laboratory (Bar Harbor, ME). 9-month-old Tg2576 mice (APP KM670/671NL) were obtained from Taconic (Rensselaer, NY). The experiment was carried out in accordance with the recommendations of “96 Guidance for Animal Experiments,” established by the “Animal Ethics Committee” at Chonnam National University, and the protocol was approved by the ‘Animal Ethics Committee’ at Chonnam National University.
2.2 Acute Restraint Stress and Hippocampal Slices Preparation
5xFAD and Tg2576 mice were physically restrained in well-ventilated 50 mL Falcon tubes for 30 min. Control mice were housed in their usual cages under normal conditions. Mice were sacrificed by cervical dislocation and then decapitated. The brains were quickly removed and submerged in ice-cold artificial cerebrospinal fluid (aCSF) containing (in mM) 124 NaCl, 3 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgSO4, and 10 glucose. The hippocampus was transversely sectioned (400-μm thick) using a McIlwain tissue chopper (Mickle Laboratory Engineering Co. Ltd.) and stabilized for 1 h via perfusion with aCSF gassed with a 95% O2/5% CO2 mixture at room temperature.
2.3 Electrophysiological Recordings
Hippocampal slices were transferred to a recording chamber perfused with oxygenated aCSF (32°C–34°C). To record field EPSP, a stimulating bipolar electrode was placed along the Schaffer-collateral pathway. Field EPSP was assessed with a glass microelectrode prepared on a micropipette puller (P-1000; Sutter Instrument, Novato, CA, USA) with 3 M NaCl (3–5 MΩ) inside. After a stable baseline was established for 30 min, LTP was induced by two tetanus stimulation strains (100 Hz for 1 s with a 30 s-interval). Field EPSP was assessed for 3 h after tetanus. Data were collected using an NI USB-6251 data acquisition module (National Instruments, Texas, USA), amplified by an Axopatch 700B amplifier (Axon Instruments, CA, USA), and using WinLTP software (http://www.winltp.com).
2.4 Golgi-Cox Staining and Spine Density Analysis
Golgi-Cox staining was performed using a FD Rapid Golgi Stain Kit (FD NeuroTechnologies Inc., USA), following the vendor's protocol. Briefly, brains were isolated and fixed in 4% paraformaldehyde for 24 h (n = 3 per group from three animals). The brain tissue was then cut into 1.5–2 mm thick slices and rinsed in 1X PBS for 5 min. Subsequently, the slices were immersed in an impregnation solution (a mixture of equal volumes of solutions A and B, prepared 24 h prior to use) and stored at room temperature for 2 weeks in the dark. Finally, the tissue was transferred into solution C and maintained at 4°C for 3 days in the dark. Afterwards, it was sliced using a McIlwain tissue chopper (Mickle Laboratory Engineering Co. Ltd.) to a thickness of 100 μm, then mounted on gelatin-coated microscope slides with a drop of solution C. After air-drying naturally at room temperature overnight, the coronal sections were immersed in the developer solution (solution D: E: double distilled water = 1:1:2) for 10 min. The sections were then dehydrated through successive steps in alcohol at increasing concentrations (50%, 75%, 95%, and 100%) before being closed with slide cover slips. Images of dendrites within the hippocampus area were captured using the CaseViewer 2.3.0 system (3DHISTECH, Budapest, Hungary). Four neurons from the CA1 region (12 neurons in total per group) displaying dendritic trees without truncations and isolated from neighboring neurons were selected. Segments of dendrites ranging from 70 to 100 μm were selected from both basal and apical dendrites of each pyramidal neuron for the analysis. The number of spines and the total length were quantified using ImageJ (NIH, Bethesda, MD, USA). The spine number was divided by the length of dendritic segments to obtain the final spine density (spines/μm) from each sample.
2.5 Measurement of ATP Concentration
After hippocampal slice preparation, the slices were incubated in oxygenated aCSF at room temperature. Hippocampal slices were collected at 100, 200, and 300 min after ARS performance, respectively. Hippocampal ATP concentrations were evaluated by an ATP bioluminescence assay kit (11699695001, Sigma-Aldrich). Hippocampal slices were homogenized with 12% perchloric acid (Sigma-Aldrich) on ice and neutralized with 30% KOH to PH 7.8, followed by centrifugation at 10,000 g for 10 min. The supernatants were collected and incubated with luciferase reagent-containing lysis buffer for 5 min. Standard curves were constructed by using known concentrations of ATP (provided with ATP bioluminescence assay kit). TD-20/20 luminometer (2020-000, Turner Designs, San Jose, CA, USA) was used for the measurement of the luminescent signal of each sample in triplicate. The level of ATP in different samples was obtained from standard curves. Furthermore, sample pellets were reconstituted with 1× PBS and used to determine the protein concentrations by using the Pierce BCA assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Divide the ATP level by the total protein amount to obtain the final ATP concentration (pmol/mg protein) from each sample.
2.6 Western Blots of Hippocampus
Hippocampus was lysed in cold RIPA buffer (AKR-190; Cell Biolabs, San Diego, CA, USA) with a protease inhibitor cocktail (210205; Cell Biolabs Inc.). Then, 30–40 μg of proteins were separated on 10%–12% SDS–polyacrylamide gel and transferred to PVDF membranes (Millipore, Bedford, MA, USA). The expressions of p-AMPK (Thr172) (#2535), total-AMPK, p-rpS6 (Ser235/236) (#2211), rpS6, p-p70S6K (Thr389) (#9205), p70S6K, or β-Actin (Cell Signaling, Danvers, MA, USA) were detected by each antibody. The immunoblots were incubated with specific secondary antibodies (Abcam) for 2 h at room temperature, and the bands were obtained using the ECL detection system (Millipore, Bedford, MA, USA).
2.7 Immunofluorescence
Mouse brain slices (20 μm) were mounted on collagen-coated glass slides (Thermo Scientific, Waltham, MA, USA), fixed in acetone solution for 10 min, washed in Tris-buffered saline, and then exposed to methanol for 5 min. Nonspecific labeling was prevented by incubating the sections in 5% bovine serum albumin (Sigma-Aldrich) for 1 h before incubating overnight at 4°C with the following specific primary antibodies (1:500 dilutions): p-AMPK (Thr172) (Invitrogen, CA, USA) and primary antiserum to NeuN (1:1000). The slices were washed 3 times (5 min each) with phosphate-buffered saline with 0.1% Tween 20 and then incubated for 2 h in the dark with AlexaFluor 488 goat anti-rabbit IgG and AlexaFluor 594 goat anti-mouse IgG (1:500; Invitrogen). All brain slices were counterstained with 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) and visualized with a confocal microscope (Carl Zeiss, Oberkochen, Germany).
2.8 Rapamycin
Rapamycin (Medchemexpress, USA) was dissolved in 100% ethanol, stored at −20°C, and freshly dissolved in an aqueous solution of 5% Tween 80 and 5% PEG 400 immediately before use. A single dose of rapamycin (40 mg/kg) was intraperitoneally injected for each mouse prior to acute restraint stress performance.
2.9 Data Analysis
Data are presented as mean ± standard error of the mean (S.E.M.). The Shapiro–Wilk test was used to determine data distribution. For comparison between two groups, an unpaired Student's t-test was used. For comparison among three or more groups, a one-way analysis of variance (ANOVA) was utilized by individual. Pearson's correlation coefficient was used to measure correlation. The value p < 0.05 was considered statistically significant.
3 Results
3.1 L-LTP Is Enhanced in the Hippocampus of Stressed 5xFAD and Tg2576 Mice
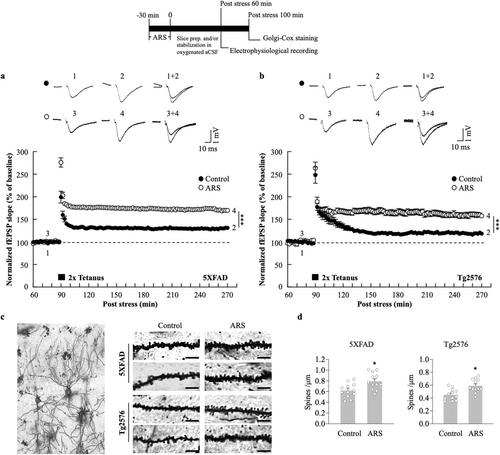
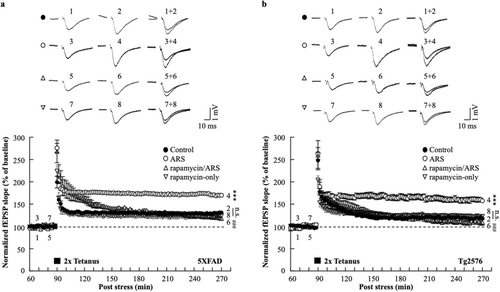
To examine the hypothesis that ARS can increase the L-LTP in the AD mouse hippocampus, we acutely prepared hippocampal slices from stressed and non-stressed 5xFAD or Tg2576 mice. Field excitatory postsynaptic field potentials (fEPSPs) were recorded in the hippocampal CA1 region and LTP was induced using an HFS protocol (two trains of 100 Hz, 100 pulses). For data acquisition, L-LTP was recorded for 180 min post-HFS. ARS exerted a sustained effect on LTP maintenance; the fEPSP slopes of L-LTP significantly increased in hippocampal slices from the stressed 5xFAD mice compared to the non-stressed mice hippocampal slices (ARS: 169.8% ± 3.1% vs. control: 131.2% ± 1.7%, p < 0.01, n = 6, Figure 1a). The enhanced fEPSP slopes were also observed in the stressed Tg2576 mice hippocampus compared to the non-stressed mice (ARS: 157.5% ± 4.3% vs. control: 118.4% ± 2.7%, p < 0.01, n = 6, Figure 1b). L-LTP is a persistent form of synaptic plasticity that requires new protein synthesis, supporting the hypothesis that it is based on the formation of new synapses [37]. To evaluate whether ARS-facilitated L-LTP was also accompanied by structural changes in the hippocampus, we performed the Golgi-Cox staining of the hippocampal CA1 pyramidal cells. The results showed an increase in dendritic spine density following ARS treatment in 5XFAD and Tg2576 mice compared to the non-stressed mice (Figure 1c,d).
3.2 Acute Restraint Stress Prevents AMPK Activation in the Hippocampus
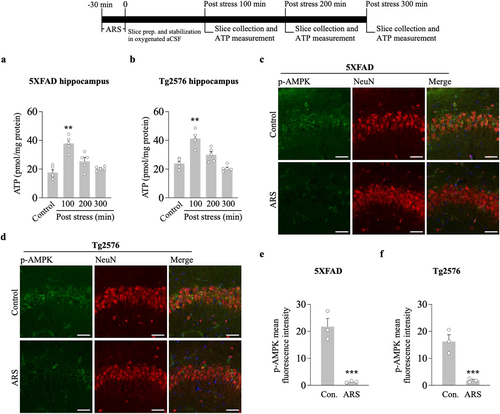
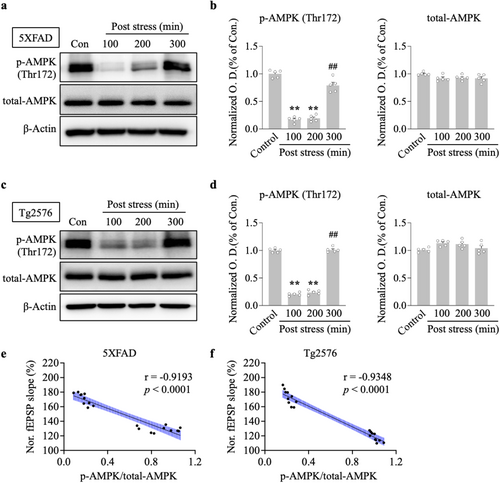
We tested the hypothesis that ARS can alter intrahippocampal ATP levels and downregulate the AMPK activity. We first measured whether 30 min of restraint stress changed the ATP concentration in the hippocampus. Hippocampal slices were analyzed for ATP concentration in stressed and non-stressed 5xFAD and Tg2576 mice. ARS for 30 min caused an increased ATP concentration in the hippocampus of the stressed group compared to the non-stressed group for 5xFAD (100 min post stress, ARS: 36.9 ± 3.3 vs. control: 17.4 ± 2.5, p < 0.01, n = 5, Figure 2a) and Tg2576 mice (100 min post stress, ARS: 41.8 ± 2.3 vs. control: 23.4 ± 1.5, p < 0.01, n = 5, Figure 2b). Next, we observed AMPK activation by measuring phosphorylated AMPKα1/2 (p-AMPK) protein levels. Immunostaining results showed that exposure to 30 min restraint stress resulted in robust inhibition of p-AMPK signals in the hippocampus compared to the non-stressed group in both 5xFAD (Figure 2c,e) and Tg2576 mice (Figure 2d,f). The inhibition of AMPK activity was accompanied by a decrease in p-AMPK protein levels at Thr172 in the hippocampus of 5xFAD (100 min vs. control, p < 0.01; 200 min vs. control, p < 0.01, n = 5, Figure 3a,b) and Tg2576 mice (100 min vs. control, p < 0.01; 200 min vs. control, p < 0.01, n = 5, Figure 3c,d). Furthermore, decreased p-AMPK levels tightly correlated with higher fEPSP slopes, as measured in the hippocampus of 5xFAD (r = −0.9193, p < 0.0001, Figure 3e) and Tg2576 mice (r = −0.9348, p < 0.0001, Figure 3f). This indicated that the time course of ARS in LTP maintenance strongly correlated with the time course of AMPK inhibition in 5xFAD mice and Tg2576 mice. These results suggest that ARS alters ATP levels, resulting in the inhibition of AMPK activity, which may play a potential role in hippocampal L-LTP enhancement.
3.3 Acute Restraint Stress Activates the mTOR Pathway
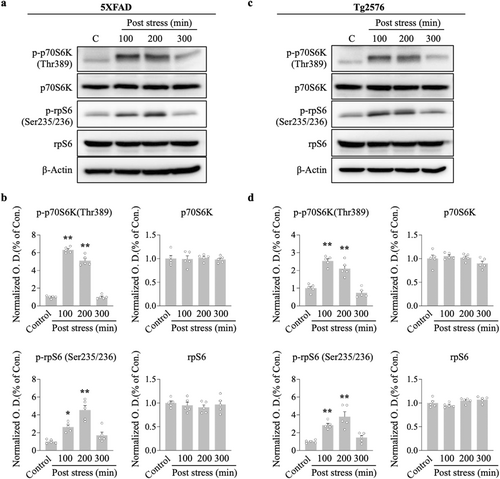
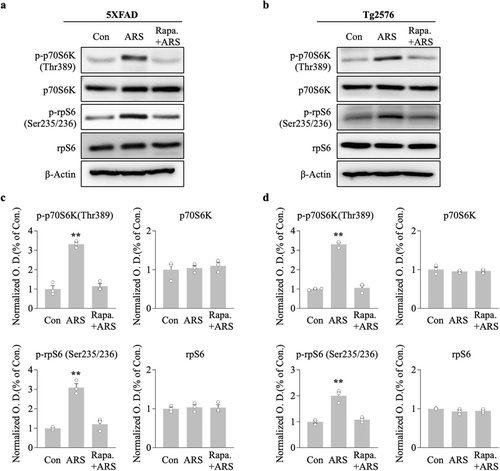
AMPK is regarded as an upstream mediator of mTOR, and the maintenance of L-LTP requires protein translation through the mTOR pathway. We conducted western blotting analysis to determine the effect of ARS on the mTOR cascade molecules, p70S6K and its substrate rpS6, because of their established role directly downstream of the mTOR kinase. ARS significantly elevated p-p70S6K and p-rpS6 protein levels in the hippocampus compared to the non-stressed group for 5xFAD (100 min vs. control, p < 0.01; 200 min vs. control, p < 0.01, n = 5, Figure 4a,b) and Tg2576 mice (100 min vs. control, p < 0.01; 200 min vs. control, p < 0.01, n = 5, Figure 4c,d). Notably, the increased effect of ARS on these protein levels persisted for more than 200 min post stress, which correlated with the time course of L-LTP maintenance in the hippocampi of the two transgenic mice.
3.4 Acute Restraint Stress Facilitation of L-LTP Is Rapamycin Sensitive
To explore the direct effect of mTOR on ARS-facilitated L-LTP in the hippocampus of AD model mice, we used a mTOR inhibitor, rapamycin (1 μM), prior to the acute stress protocol. The effect of rapamycin on the L-LTP was assessed using electrophysiological recordings of the hippocampal CA1 region. In hippocampal slices from stressed mice, the fEPSP slope of the L-LTP was significantly blocked by rapamycin compared to the ARS-only group for 5xFAD (rapamycin/ARS: 117.8% ± 1.3% vs. ARS: 169.8% ± 3.1%, p < 0.001, n = 6, Figure 5a) and Tg2576 mice (rapamycin/ARS: 109.2% ± 6.4% vs. ARS: 157.5% ± 4.3%, p < 0.001, n = 6, Figure 5b). Rapamycin administered prior to the electrophysiological recording did not affect L-LTP levels, as indicated by a similar fEPSP slope in rapamycin and control slices for 5xFAD (rapamycin-only: 123.9% ± 4.6% vs. control: 131.2% ± 1.7%, n.s., n = 6, Figure 5a) and Tg2576 mice (rapamycin-only: 122.6% ± 3.7% vs. control: 118.4% ± 2.7%, n.s., n = 6, Figure 5b). ARS failed to enhance L-LTP levels in the presence of rapamycin, suggesting that ARS-mediated L-LTP requires the mTOR pathway. To further investigate this, we examined the effect of rapamycin on the ARS-triggered mTOR pathway in the hippocampi of AD mouse models. We analyzed the phosphorylation of the mTOR cascade components p70S6K and rpS6, and western blotting of hippocampal proteins showed that rapamycin eliminated the ARS-induced activation of the mTOR pathway in rapamycin-pretreated mice compared to untreated mice (Figure 6). Taken together, these results suggested that ARS regulates AD mouse hippocampal ATP production and inhibits AMPK activity, which mediates the mTOR pathway involved in the maintenance of L-LTP.
4 Discussion
We first investigated whether 30 min of ARS facilitates a long-lasting form of synaptic plasticity, termed L-LTP, in the hippocampal CA1 region of 5xFAD and Tg2576 mice. Second, we highlighted that ARS regulates ATP concentration and significantly inhibits AMPK activity. Importantly, the time course of AMPK phosphorylation strongly correlated with the time course of L-LTP maintenance by ARS, suggesting a potential connection between AMPK inhibition and L-LTP maintenance. Third, ARS activated the mTOR pathway through increased phosphorylation of the mTOR components p70S6K and rpS6. Furthermore, rapamycin prevented the enhancement of L-LTP, suggesting that acute stress inhibits AMPK activity and regulates the mTOR pathway, resulting in the maintenance of L-LTP in the hippocampus of AD model mice.
Acute stress produces adaptive effects in humans, including enhanced selective attention [38, 39] and improved working memory [40, 41]. Patients with AD exhibit a prominent loss of hippocampus-dependent memory. Hippocampal synaptic impairment is considered an early alteration in patients with early-stage AD [42, 43]. One form of prolonged increased synaptic efficacy is LTP, which is widely regarded as the synaptic basis for information storage in the mammalian brain [44]. Many studies have reported a reduction in LTP in AD model mice, such as 5xFAD [45] and Tg2576 mice [46], which develop an age-dependent motor phenotype in addition to working memory deficits. Therefore, measuring hippocampal synaptic plasticity in AD mouse models serves as a useful indicator of AD-related synaptic degeneration and memory loss. It has been shown that acute stress or emotional events can facilitate hippocampal LTP [33, 35]. In line with this, our previous study demonstrated that 30 min of ARS rescued impaired LTP in the hippocampus of AD model mice, particularly through glucocorticoid-mediated effects on hippocampal function, focusing on LTP-related glutamatergic receptor trafficking and its role in impaired synaptic plasticity [36]. Consistent with these findings, we observed a long-lasting enhancement of synaptic plasticity in the hippocampal CA1 area in both 5xFAD and Tg2576 mice following ARS. These findings suggest that ARS-mediated L-LTP maintenance plays a beneficial role in regulating information storage in AD-related pathologies. Additionally, given that the effect of ARS appears to be time-limited in AD-related pathologies, further studies are needed to develop methods to prolong or optimize this synaptic enhancement, which could have significant clinical applications.
AMPK is an important modulator of cellular energy homeostasis, and its activity is regulated by low neuronal energy [47]. AD has been reported to exert abnormal energy metabolism in neurons [48]. In addition, highly activated AMPK has been observed in AD mice and AD brains [49, 50]. Recently, the AMPK pathway has received increasing attention in neuronal therapeutic research. Several studies have reported that inhibition of AMPK activity has a neuroprotective effect [51, 52]. In the current study, ARS strongly inhibited the phosphorylation of AMPK at Thr172 in the hippocampus of two AD mouse models. These findings highlight the beneficial role of ARS in alleviating AD-related pathologies by suppressing AMPK activity. AMPK also regulates protein synthesis, which plays an important role in enhancing synaptic plasticity and memory [53]. A previous study reported that pharmacological inhibition of AMPK activity increased L-LTP maintenance [25]. Consistently, we found that the time course of the AMPK phosphorylation strongly correlated with the time course of L-LTP maintenance by ARS, suggesting a potential connection between AMPK inhibition and synaptic plasticity improvement under AD conditions.
mTOR downstream substrates are highly expressed in the postsynapse, indicating a local function of mTOR in synaptic plasticity. Studies linking the mTOR pathway to synaptic plasticity were first conducted in invertebrates using rapamycin [54]. Furthermore, L-LTP requires local protein synthesis, which is disrupted in the rat hippocampus upon exposure to rapamycin [11, 55]. AMPK is a regulator of the mTOR pathway and directly affects L-LTP maintenance. AMPK activation prevents the L-LTP expression that is increased by AMPK inhibitors. Moreover, rapamycin has been shown to completely block the effect of an AMPK inhibitor on L-LTP enhancement [25]. These reports have demonstrated the critical role of the mTOR pathway in regulating synaptic plasticity. In our study, L-LTP in stressed mice resulted in long-lasting enhancement of the hippocampal CA1 region. Rapamycin blocked these changes in the maintenance of L-LTP in the hippocampi of stressed mice.
Previous studies have suggested that physical exercise reduces depressive behaviors, enhances hippocampal-dependent learning, increases hippocampal neurogenesis, and synaptic plasticity [56, 57]. In fact, the therapeutic effects of physical exercise in treating AD are increasingly recognized [58, 59]. Various mechanisms have been proposed for the beneficial properties of physical exercise in AD, including a reduction in abnormal protein aggregates and neuroinflammation, and a boost in neurogenesis [60]. It was shown that acute stress and exercise share common neural mechanisms [61, 62]. Indicating that appropriately managed acute stress stimulation during daily activities could be beneficial for patients with AD. Moreover, identifying these underlying mechanisms could help promote the development of novel pharmaceutical agents in the treatment of AD. We acknowledge the limitations of the current study, particularly in terms of the behavioral testing used to assess cognitive function. Future studies will explore alternative approaches to extend the duration of the ARS effect, such as repeated physical activity in AD mice, to better accommodate experimental constraints.
5 Conclusion
Here, using 5xFAD and Tg2576 mice, we directly measured the hypothesis that acute restraint stress increases hippocampal ATP production, then suppresses the aberrant AMPK activity and removes the inhibition of the mTOR pathway, which rescues the AD-induced synaptic plasticity impairment (Figure 6). Thus, the acute stress mediating the AMPK-mTOR pathway is likely to be a beneficial candidate to enhance synaptic functions under AD-related pathologies.
Author Contributions
Ming Wang: conceptualization, data curation, methodology, investigation, writing – original draft, supervision, funding acquisition. Baoyuan Jin: data curation, methodology, investigation, formal analysis, validation, resources, writing – review and editing. Jihoon Jo: methodology, supervision, funding acquisition.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




