The Relationship Between the Average Infusion Rate of Propofol and the Incidence of Delirium During Invasive Mechanical Ventilation: A Retrospective Study Based on the MIMIC IV Database
Funding: This work was supported by the Medical Scientific Research Project of Jiangsu Health Commission (ZD2021011) and the National Natural Science Foundation of China (82002454 and 82273325).
Qi-Yue Ge and Chao Zheng contributed equally to this study.
ABSTRACT
Background
Delirium is a common complication observed in intensive care units (ICUs). Propofol is one of the most widely used sedatives and is believed to be closely connected with the incidence of delirium. The study was carried out to explore the relationship between delirium and the average rate of propofol infusion.
Methods
Patients who underwent invasive mechanical ventilation (IMV) while receiving propofol from the Medical Information Mart for Intensive Care IV (MIMIC IV) database were included in the study. The primary outcome was to identify the potential risk factors for the incidence of delirium and investigate the relationship between the average rate of propofol infusion and the incidence of delirium. The secondary outcome was to further analyze the relationship by subgroup analysis. Propensity score matching (PSM) was employed to minimize bias.
Results
A total of 16,956 patients (delirium: 5805; control: 11,151) were ultimately included in the study after PSM. The median diagnostic time of delirium was 18 h. An average propofol infusion rate ≥ 20 μg/(kg*h) during the initial 18 h was found to be independently significant [OR = 1.84, 95% CI = (1.72, 1.98), p < 0.001], while an average propofol infusion rate ≤ 40 μg/(kg*h) in the first hour showed no statistically significant difference in the incidence of delirium [OR = 0.95, 95% CI = (0.88, 1.02), p = 0.163]. Besides, an average propofol infusion rate ≥ 20 μg/(kg*h) was also found to be statistically significant in all the subgroup analyses.
Conclusion
An average propofol infusion rate ≥ 20 μg/(kg*h) during the initial 18 h was identified as an independent risk factor for delirium, suggesting that the accumulation of propofol might be associated with an increased incidence of delirium.
Abbreviations
-
- BIDMC
-
- Beth Israel Deaconess Medical Center
-
- CAM-ICU
-
- Confusion Assessment Method for the Intensive Care Unit
-
- GCS
-
- Glasgow Coma Scale
-
- ICU
-
- intensive care unit
-
- IMV
-
- invasive mechanical ventilation
-
- LODS
-
- logistic organ dysfunction system
-
- MIMIC IV
-
- Medical Information Mart for Intensive Care IV
-
- OASIS
-
- Oxford Acute Severity of Illness Score
-
- OR
-
- odds ratio
-
- PSM
-
- propensity score matching
-
- RCS
-
- restricted cubic splines
-
- SAPS II
-
- Simplified Acute Physiology Score II
-
- SIRS
-
- systemic inflammatory response syndrome
-
- SOFA
-
- sequential organ failure assessment
1 Introduction
Delirium, known as an acute confusion state, is a prevalent complication during invasive mechanical ventilation (IMV) [1]. Several factors contribute to the incidence of delirium, including advanced age, exposure to infection, and the use of certain drugs, so that all patients have the risk for delirium. Patients in intensive care units (ICUs), who are typically among the most critically ill in the hospital, might be at an even higher risk for delirium. Sedation is necessary during IMV, and guidelines recommend the use of nonbenzodiazepine sedatives, such as propofol, for critically ill patients undergoing IMV [2]. Propofol, which acts on gamma-aminobutyric acid receptors, is widely used for sedation and is employed in the induction and maintenance of general anesthesia, as well as in the treatment of delirium due to its notable efficacy [3]. However, propofol was reported to have a potential association with the incidence of delirium [4-6]. Despite this, the mode by which propofol may induce delirium remains unclear. Therefore, it is important to investigate the relationship between delirium and propofol.
Delirium, characterized by an acute change in mental status, poses significant diagnostic challenges. Many researches have demonstrated that in-hospital delirium is associated with poor prognosis and reduced postdischarge life quality [7]. Thus, identifying high-risk patients is crucial for the early detection and management of delirium.
Previous studies have primarily concentrated on comparing propofol with other sedatives, such as midazolam or dexmedetomidine, with limited attention devoted to the correlation between the rate of propofol infusion and the incidence of delirium. The study was launched to identify the high delirium-risk patients and explore potential strategies to reduce the incidence of delirium.
2 Methods and Materials
2.1 Study Design
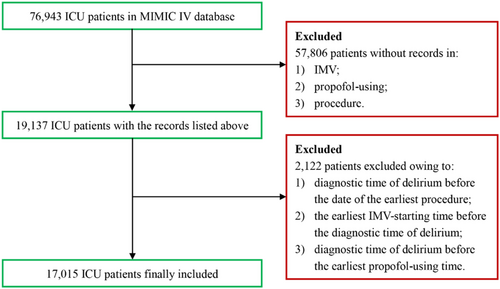
The retrospective study was performed by using data from the Medical Information Mart for Intensive Care IV (MIMIC IV) [8]. MIMIC IV database (version 2.2) contains 76,943 ICU patients between 2008 and 2019 from the Beth Israel Deaconess Medical Center (BIDMC) in Boston, USA.
2.2 Patients
ICU patients from BIDMC (via the MIMIC IV database) from 2008 to 2019 were included. The inclusion criteria were listed as follows: (1) ICU patients underwent IMV; (2) patients with records in procedures_icd table; and (3) patients with propofol-using records in hospital. The exclusion criteria were as follows: (1) diagnostic time of delirium before the date of the earliest procedure; (2) the earliest IMV-starting time before the diagnostic time of delirium; and (3) diagnostic time of delirium before the earliest propofol-using time.
2.3 Data Collection
Delirium patients were diagnosed by CAM-ICU score. Patients who satisfied feature 1 + 2 + 3 or 1 + 2 + 4 according to the CAM-ICU scoring system were classified as delirium patients. Patients with IMV-using were identified by the records of ventilation status. Propofol was distinguished by item ID in “222168.” We defined the average rate of propofol infusion as (total dosage of propofol infusion during the duration/length of the duration).
Data collected from the MIMIC IV database included baseline characteristics included were list as follows: age, gender (male and female), ethnicity (white, black, and other), last care unit (TSICU, MICU/SICU, NICU, CVICU, and CCU), first-day SOFA, first-day GCS, SIRS, LODS, OASIS, and SAPS II. The relationship between average rate of propofol infusion and delirium was the primary outcome of the study. Secondary outcomes were in-hospital mortality and the difference between the high-delirium risk group and the low-delirium risk group.
2.4 Ethics
We have completed the National Institutes of Health's web-based course and passed the Protecting Human Research Participants exam (ID: 52932612) to access the MIMIC IV database.
2.5 Statistical Analyses
Selected data from the MIMIC IV database was exported as a .xlsx file and then transferred into a .dta file. Stata/SE 16.0 software (Stata Corp, College Station, TX, USA) and R 4.3.3 software (The R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org) were employed to analyze the data. Categorical variables were analyzed by Chi-squared test and Fisher's exact test. Normally distributed continuous variables were analyzed by t-test and the non-normal distributed variables were compared by Kruskal–Wallis H test. Restricted cubic splines (RCS) were carried out to delineate the potential threshold for a high-risk amount of propofol. Multiple logistic regression was applied to further analyze the association between characteristics and high-risk amounts of propofol. A small amount of missing data existed and was replaced by the median. PSM was performed to reduce the bias and the standardized difference figure was used to evaluate the effect of PSM. p-value < 0.05 was considered to be significantly different.
3 Results
3.1 Baseline Characteristics
The flowchart of the inclusion is depicted in Figure 1. A comprehensive sample of 17,015 patients (delirium: n = 5811; control: n = 11,204) was enrolled. Baseline characteristics, including gender, ethnicity, last care unit, first-day SOFA, first-day GCS, SIRS, LODS, OASIS, and SAPS II, and clinical information are summarized in Table 1. To minimize the potential bias of the study, PSM was employed to adjust for disparities in baseline characteristics. A refined sample of 16,956 patients was ultimately included after PSM (delirium: n = 5805; control: n = 11,151). Group demographics and cohort characteristics are listed in Table S1. The assessment of PSM performance is presented in Figure S1.
| Delirium (n = 5811) | Control (n = 11,204) | p | |
|---|---|---|---|
| Age, year | |||
| Median, IQR | 66.32 (54.89, 75.99) | 65.91 (55.29, 75.61) | 0.442 |
| Gender | |||
| Female | 2380 | 4165 | < 0.001 |
| Male | 3431 | 7039 | |
| Ethnicity | |||
| White | 3533 | 7664 | < 0.001 |
| Black | 584 | 796 | |
| Other | 1694 | 2744 | |
| Last care unit | |||
| TSICU | 912 | 1548 | < 0.001 |
| MICU/SICU | 2899 | 3923 | |
| NICU | 459 | 147 | |
| CVICU | 1151 | 5137 | |
| CCU | 390 | 449 | |
| First-day SOFA | |||
| Median, IQR | 7 (5, 11) | 5 (3, 8) | < 0.001 |
| First-day GCS | |||
| Median, IQR | 12 (8, 14) | 14 (10, 15) | < 0.001 |
| SIRS | |||
| Median, IQR | 3 (2, 3) | 3 (2, 3) | 0.807 |
| LODS | |||
| Median, IQR | 7 (5, 9) | 5 (3, 8) | < 0.001 |
| OASIS | |||
| Median, IQR | 39 (34, 45) | 36 (31, 41) | < 0.001 |
| SAPS II | |||
| Median, IQR | 41 (32, 51) | 37 (31, 47) | < 0.001 |
| Average propofol infusion rate, μg/(kg*h) | |||
| 1 h | |||
| Median, IQR | 37.65 (22.71, 48.92) | 40.76 (29.35, 51.78) | < 0.001 |
| 2 h | |||
| Median, IQR | 36.50 (21.41, 48.62) | 40.16 (29.09, 50.98) | < 0.001 |
| 3 h | |||
| Median, IQR | 34.81 (20.70, 47.80) | 39.04 (25.55, 50.04) | < 0.001 |
| 4 h | |||
| Median, IQR | 32.94 (20.24, 46.52) | 35.53 (22.69, 48.09) | < 0.001 |
| 5 h | |||
| Median, IQR | 31.57 (19.98, 45.24) | 32.38 (20.30, 45.59) | 0.017 |
| 6 h | |||
| Median, IQR | 30.49 (19.35, 43.93) | 30.08 (18.79, 43.24) | 0.220 |
| 7 h | |||
| Median, IQR | 29.96 (18.44, 42.85) | 28.30 (17.01, 41.48) | < 0.001 |
| 8 h | |||
| Median, IQR | 29.38 (17.51, 42.22) | 26.32 (15.45, 40.33) | < 0.001 |
| 9 h | |||
| Median, IQR | 28.69 (16.89, 41.47) | 24.75 (14.14, 39.46) | < 0.001 |
| 10 h | |||
| Median, IQR | 28.07 (16.15, 40.78) | 23.25 (13.02. 38.28) | < 0.001 |
| 11 h | |||
| Median, IQR | 27.41 (15.45, 40.32) | 21.88 (12.00, 37.11) | < 0.001 |
| 12 h | |||
| Median, IQR | 26.92 (14.91, 39.99) | 20.79 (11.13, 36.06) | < 0.001 |
| 18 h | |||
| Median, IQR | 23.74 (11.99, 36.75) | 15.71 (7.79, 30.08) | < 0.001 |
| 24 h | |||
| Median, IQR | 21.08 (10.11, 33.76) | 12.32 (5.90, 25.33) | < 0.001 |
| 30 h | |||
| Median, IQR | 19.08 (8.52, 31.78) | 10.12 (4.77, 21.53) | < 0.001 |
| 36 h | |||
| Median, IQR | 17.17 (7.42, 29.78) | 8.53 (4.00, 18.82) | < 0.001 |
| 42 h | |||
| Median, IQR | 15.55 (6.52, 27.81) | 7.38 (3.44, 16.63) | < 0.001 |
| 48 h | |||
| Median, IQR | 14.05 (5.83, 26.33) | 6.50 (3.02, 14.77) | < 0.001 |
| In-hospital mortality | |||
| Yes | 953 | 1452 | < 0.001 |
| No | 4858 | 9752 | |
3.2 Primary Outcomes
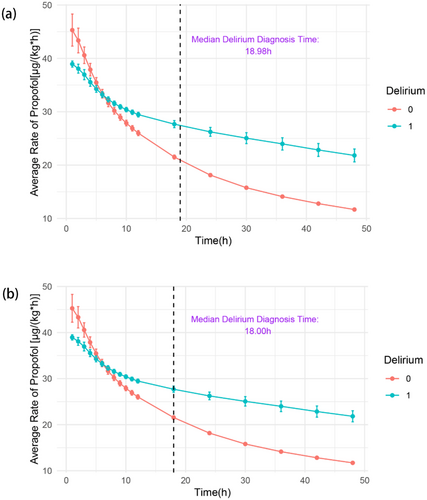
The disparities in the average propofol infusion rate between the delirium and control groups are graphically presented in Figure 2. Patients belonging to the delirium group exhibited a notably higher average propofol infusion rate in the initial 5 h of IMV, followed by a decrease to a lower rate subsequent to 7 h. Specifically, the median time to diagnose delirium was the 18th hour.
Following the application of RCS, 40 μg/(kg*h) within the first hour and 20 μg/(kg*h) within the initial 18 h were selected as the indicators to differentiate between high and low risks of delirium, respectively (Figure S2a and Figure 3). Additionally, stacked bar charts depicting these data are also shown in the figures (Figure S2b and Figure 3b). A higher proportion of delirium patients was observed in the group that received a higher average rate of propofol infusion.
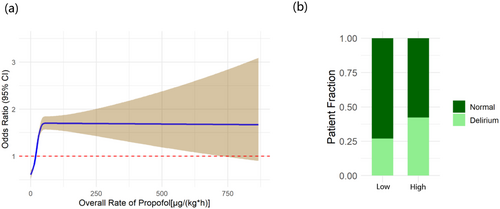
In the multiple logistic regression model, a higher incidence of delirium was observed to be associated with an average propofol infusion rate exceeding 20 μg/(kg*h) during the initial 18 h. However, an average propofol infusion rate below 40 μg/(kg*h) within the first hour did not demonstrate any statistically significant difference in the incidence of delirium. Multicollinearity was also rigorously assessed, and the variables were appropriately adjusted accordingly (Tables S2 and S3). Notably, the results remained consistent in both 18 h model [OR = 1.84, 95% CI = (1.72, 1.98), p < 0.001] and 1 h model [OR = 0.95, 95% CI = (0.88, 1.02), p = 0.163] after adjustment (Table 2 and Table S4).
| Delirium | OR | Standard error | z | p > |z| | 95% CI |
|---|---|---|---|---|---|
| Age, years | |||||
| (< 60) | |||||
| ≥ 60 | 1.318194 | 0.0506589 | 7.19 | < 0.001 | [1.222551, 1.421318] |
| Gender | |||||
| (Female) | |||||
| Male | 0.9047045 | 0.0327836 | −2.76 | 0.006 | [0.8426785, 0.9712958] |
| Race | |||||
| (Other) | |||||
| White | 0.8371806 | 0.0339406 | −4.38 | < 0.001 | [0.7732326, 0.9064172] |
| Black | 1.092213 | 0.0741012 | 1.30 | 0.194 | [0.9562191, 1.247547] |
| Last care unit | |||||
| (TSICU) | |||||
| MICU/SICU | 1.013774 | 0.0515744 | 0.27 | 0.788 | [0.9175658, 1.120069] |
| NICU | 6.082144 | 0.6461928 | 16.99 | < 0.001 | [4.9388, 7.490175] |
| CVICU | 0.3574196 | 0.020043 | −18.35 | < 0.001 | [0.3202179, 0.3989432] |
| CCU | 1.122617 | 0.095265 | 1.36 | 0.173 | [0.9506026, 1.325759] |
| First-day SOFA | 1.126682 | 0.0051812 | 25.94 | < 0.001 | [1.116572, 1.136883] |
| High-risk (18 h), μg/(kg*h) | |||||
| (< 20) | |||||
| ≥ 20 | 1.844288 | 0.0656746 | 17.19 | < 0.001 | [1.719957, 1.977606] |
| Constant | 0.2103473 | 0.0142313 | −23.04 | < 0.001 | [0.1842247, 0.240174] |
3.3 Secondary Outcomes
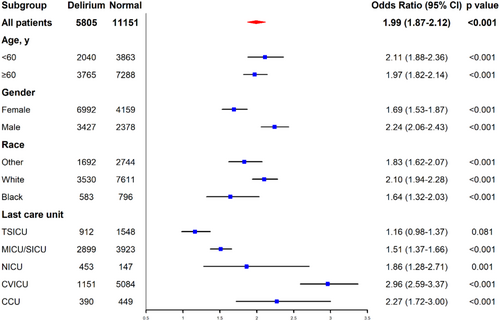
Subgroup analysis of the logistic regression revealed that average propofol amount exceeding 20 μg/(kg*h) had close connection in specific characteristics of the population, including age (< 60) [OR = 2.11, 95% CI = (1.88, 2.36), p < 0.001], age (≥ 60) [OR = 1.97, 95% CI = (1.82, 2.14), p < 0.001], gender (female) [OR = 1.69, 95% CI = (1.53, 1.87), p < 0.001], gender (male) [OR = 2.24, 95% CI = (2.06, 2.43), p < 0.001], race (white) [OR = 2.10, 95% CI = (1.94, 2.28), p < 0.001], race (black) [OR = 1.64, 95% CI = (1.32, 2.03), p < 0.001], race (other) [OR = 1.83, 95% CI = (1.62, 2.07), p < 0.001], last care unit (TSICU) [OR = 1.16, 95% CI = (0.98, 1.37), p = 0.081], last care unit (MICU/SICU) [OR = 1.51, 95% CI = (1.37, 1.66), p < 0.001], last care unit (NICU) [OR = 1.86, 95% CI = (1.28, 2.71), p = 0.001], last care unit (CVICU) [OR = 2.96, 95% CI = (2.59, 3.37), p < 0.001], and last care unit (CCU) [OR = 2.27, 95% CI = (1.72, 3.00), p < 0.001]. The forest plot is shown in Figure 4.
4 Discussion
The results indicated that patients with delirium had a higher average rate of propofol infusion during the first 18 h, suggesting that an average propofol infusion rate of ≥ 20 μg/(kg*h) in the initial 18 h was a significant risk factor for delirium. The hypothesis was supported by the multiple logistic regression model. Conversely, although Figure 2 demonstrates differences both at the first hour and during the initial 18 h, the average propofol infusion rate under 40 μg/(kg*h) in the first hour was found not to be associated with the higher incidence of delirium in another multiple logistic regression model. The findings suggested that the accumulation of propofol, rather than a higher momentary rate, might be the actual contributing factor to the increased incidence of delirium. A similar result was observed in a previous study on anesthesia induction. No significant difference in delirium incidence was found between varying propofol injection rates [9]. Additionally, as depicted in Table 1, the incidence of delirium was connected to a higher in-hospital mortality (p < 0.001), indicating that reducing the accumulation of propofol might potentially decrease the in-hospital mortality.
The results indicated that, in addition to the average propofol infusion rate, age, gender, race, last care unit, and first-day SOFA were also associated with the incidence of delirium. Previous studies have generally identified the elderly as a risk factor for delirium [10, 11]. The SOFA score was designed to evaluate the degree of organ dysfunction or failure and the current research depicts that the SOFA score is a risk factor for both delirium and in-hospital mortality [12]. Besides, gender was also recognized as an important factor in the incidence of delirium in prior studies. An investigation in the relationship between delirium and gender found that delirium would more frequently affect female patients over 85 years old in the cardiac ICU, which might explain the difference in gender observed in the multiple logistic regression model [13]. Additionally, patients in the NICU exhibited a higher incidence of delirium. One potential explanation was that the neurological disease itself could result in delirium by inducing neurological inflammation and other mechanisms, making NICU residency a significant factor in the incidence of delirium [14, 15]. In contrast to the findings in cardiovascular surgery [16-18], although patients in CVICU showed similar outcomes as other ICUs during subgroup analysis, CVICU appeared to have a lower impact on delirium compared to other ICUs. This phenomenon might be attributed to the low proportion of patients who underwent extracorporeal circulation and open-heart operation.
To deal with the high incidence of delirium associated with propofol, several researches paid attention to the alternatives. Dexmedetomidine has also been widely utilized in patients undergoing IMV. Evidence-based studies revealed that dexmedetomidine might shorten the duration of IMV and reduce the incidence of delirium [4, 5]. However, dexmedetomidine might also increase the risk of cardiac events [19]. Another commonly used sedative was midazolam. Previous researches depicted that although midazolam exhibited similar sedative efficacy, it was associated with higher mortality and an increased incidence of delirium [20, 21]. Future studies should focus on alternative sedatives with both a lower incidence of delirium and safety.
In the treatment of delirium, pharmacological intervention was generally considered as a common practice in delirium treatment. However, recent researches demonstrated that drugs like haloperidol and other antipsychotics might not be as effective as expected. Prevention was considered to be the most optimal treatment. Nonpharmacologic interventions have been important approaches in the past few decades and were recommended by clinical guidelines [14, 22, 23]. Future treatment for delirium should concentrate on the combination of pharmacologic therapies, nonpharmacologic prevention, and monitoring.
A key strength of our study was that it was the first study to concentrate on the relationship between average propofol infusion rate and the incidence of delirium in patients undergoing IMV. Furthermore, the research included a relatively large sample size. The results of this study suggested that the role of the average propofol infusion rate during the initial 18 h made great sense to the incidence of delirium, which might contribute to the future management of ICU patients undergoing IMV.
Some limitations also existed in the study. Initially, the average rate of propofol infusion was calculated based on the weight, time, and total amount of propofol, which made it difficult to explore the relationship between delirium and temporary propofol infusion rate. Secondarily, the effects of other drugs were not comprehensively considered, though the influence of propofol was demonstrated by multiple logistic regression. Additionally, it was difficult to identify different subtypes of delirium within the MIMIC IV database, which should be taken into consideration in future studies.
5 Conclusion
An average propofol infusion rate ≥ 20 μg/(kg*h) during the initial 18 h was an independent risk factor for delirium, suggesting that the accumulation of propofol might be associated with an increased incidence of delirium. Future studies should focus on the different subtypes of delirium and comprehensive management of delirium.
Author Contributions
Qi-Yue Ge, Chao Zheng, and Yi Shen completed the conceptualization. The methodology was finished by Qi-Yue Ge, Chao Zheng, Zhuang-Zhuang Cong, Yan-Qing Wang, Bing-Wei Chen, and Yi Shen. The formal analysis was accomplished by Qi-Yue Ge and Chao Zheng. The investigation was contributed by Qi-Yue Ge, Chao Zheng, Jing Luo, Xiao-Bin Song, Hao-Tian Zheng, and Peng-Long Zhao. The data curation was done by Qi-Yue Ge and Chao Zheng. The visualization was achieved by Qi-Yue Ge and Chao Zheng. Zhuang-Zhuang Cong, Jing Luo, and Yi Shen made great contributions to the funding acquisition. Resources were completed by Qi-Yue Ge, Chao Zheng, and Yi Shen. Supervision was given by Yan-Qing Wang, Bing-Wei Chen, and Yi Shen. All authors wrote and/or reviewed the manuscript.
Acknowledgments
The authors have nothing to report.
Ethics Statement
We have completed the National Institutes of Health's web-based course and passed the Protecting Human Research Participants exam (ID: 52932612) to access the MIMIC IV database; Due to the retrospective nature, the study was dispensed from obtaining individual inform consents.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Dataset of the study could be obtained from the MIMIC IV database (https://mimic.mit.edu).




