Effects of Transcranial Direct Current Stimulation Targeting Dorsolateral Prefrontal Cortex and Orbitofrontal Cortex on Somatic Symptoms in Patients With Major Depressive Disorder: A Randomized, Double-Blind, Controlled Clinical Trial
Funding: This study was supported by the Shanghai “Science and Technology Innovation Action Plan” Medical Innovation Research (21Y11905600); Shanghai “Science and Technology Innovation Action Plan” Natural Science Foundation of Shanghai (21ZR1455100); Science and Technology Innovation Project of Shanghai Jiao Tong University School of Medicine (Humanities and Social Sciences) (No. WK2017); STI2030-Major Projects (2021ZD0200800); National Key R&D Program of China (2022YFC3302001); and Research Physician Program of Shanghai Mental Health Center (2021-YJXYS-06).
ABSTRACT
Aim
There is a lack of research on transcranial direct current stimulation (tDCS) for the treatment of somatic symptoms in major depressive disorder (MDD) and the suitable stimulating brain region. We investigated the efficacy of tDCS targeting the dorsolateral prefrontal cortex (DLPFC) versus orbitofrontal cortex (OFC) on depressive somatic symptoms and somatic anxiety in patients with MDD and aimed to identify the appropriate stimulating brain regions.
Methods
In this randomized, double-blind, sham-controlled study, a total of 70 patients diagnosed with MDD were randomly allocated into DLPFC group, OFC group, and Sham group. Subjects participated in 2 weeks of 10 primary interventions and subsequently 2-week maintenance interventions weekly (20 min, 2 mA).
Results
The DLPFC group showed a more significant improvement in somatic symptoms compared to the Sham group at week 2. At the maintenance and follow-up stages, the DLPFC group outperformed the Sham and OFC groups, but the difference with the Sham group was not significant. Neither active group demonstrated superiority over the Sham group in improving depression and anxiety.
Conclusion
In conclusion, the tDCS targeting DLPFC may be a potentially effective therapeutic target for alleviating somatic symptoms in patients with MDD.
1 Introduction
It is estimated that approximately 3.8% of the global population, equating to around 280 million individuals, are afflicted with major depressive disorder (MDD) [1]. The Global Burden of Disease Study identifies MDD as a leading contributor to worldwide disease burden, underscoring the urgency of effective treatment strategies [2].
Patients diagnosed with MDD frequently exhibit somatic symptoms, which are primarily categorized into vegetative, pain, and non-pain symptoms. Vegetative symptoms encompass sleep disturbances, changes in appetite, and lack of energy. Pain symptoms include headache, back pain, gastrointestinal disturbances, and musculoskeletal pain. Non-pain symptoms comprise dizziness, palpitations, dyspnea, and shortness of breath [3-5]. Moreover, somatic anxiety is prevalent among MDD patients and is linked to the response to antidepressant treatment [6]. An international multicenter study revealed that approximately two-thirds of MDD patients exhibit somatic symptoms [7]. Furthermore, these somatic symptoms often coexist with depression and anxiety [8]. The concurrent presence of somatic symptoms and mental health issues amplifies the disease burden by impacting health outcomes, functionality, and economic costs [9-11]. Antidepressants are the most frequently prescribed medication for treating somatic symptoms. However, these symptoms have shown less responsiveness to conventional antidepressant treatment compared to depressive symptoms [12, 13]. Therefore, it is imperative to explore effective interventions to address somatic symptoms in MDD patients.
In recent years, innovative noninvasive transcranial brain stimulation techniques such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) have been advanced for depression treatment [14-16]. Compared to rTMS, tDCS treatments are more cost-effective and can be implemented in diverse environments, including home-based care [17]. The tDCS technique injects a weak direct current through scalp electrodes, and the current passes through several anatomical layers to the brain, where it modulates neuronal excitability and cortical activity via changing the membrane potential according to stimulation parameters [18, 19]. Meta-analysis based primarily on studies of patients with combination therapy, with or without medication, and with unipolar or bipolar depression has shown that tDCS can yield low to moderate antidepressant effects [20, 21]. Meanwhile, a recent meta-analysis revealed that the impact of tDCS monotherapy was substantial but exhibited considerable heterogeneity [22]. It has also been suggested that active tDCS is not superior to sham tDCS in the treatment of unipolar or bipolar depression [23]. Although tDCS is one of the promising treatments for MDD according to evidence-based guidelines, the optimal protocol is still worth exploring [24].
Studies of tDCS treating MDD typically position anodes on the hypoactive left dorsolateral prefrontal cortex (DLPFC). This positioning aims to enhance local activity and restore normal function [25]. Evidence indicates that tDCS treatment, when targeting the DLPFC, is not only effective in addressing acute depressive episodes but also beneficial in managing the follow-up of depression, suggesting that tDCS intervention may augment the benefits derived from the acute treatment phase [26]. Cathode placement on the right DLPFC or right supraorbital or frontotemporal regions results in different current distributions, which explains the various outcomes of different stimulation protocols [27]. Due to the imbalance of left–right DLPFC in MDD characterized in neuropsychological regard, research found that one rationale for placing electrodes over DLPFC in depression is improving dysfunctional cognition underlying negative mood in depression [28, 29]. The orbitofrontal cortex (OFC) has been consistently involved in neural systems important for emotion regulation and stress response, as well as in neural markers of outcome and treatment response in depression [30]. A review of OFC corticostriatal circuits suggests that the OFC circuitry could be a promising target for therapeutic brain stimulation in psychiatric disorders [31]. Furthermore, research has found that rTMS treatment using inhibitory stimulation of the right OFC improves depressive symptoms in patients with MDD comorbid with obsessive–compulsive disorder [32, 33]. Nonetheless, there is a paucity of studies investigating cathode-targeted OFC-tDCS for depression. Therefore, it is necessary to further investigate the efficacy of tDCS with different targets in treating depression.
In addition to regulating the mood of MDD patients, tDCS can also treat pain symptoms, which are one of the core symptoms of somatic symptoms [34]. The DLPFC plays a crucial role in pain management by modulating both cortico-subcortical and cortico-cortical pathways, involving the engagement of both somatosensory regions and regions responsible for processing emotionally significant stimuli [35]. The most commonly used tDCS treatment paradigm for pain control is anodal stimulation of the left DLPFC [36]. Additionally, tDCS protocols involving anodal stimulation of the DLPFC have proven beneficial in treating insomnia and sleep disturbances in neuropsychiatric populations [37]. Research has also revealed a correlation between the OFC and somatic symptoms. Specifically, more somatic symptoms are associated with less surface area of left OFC [38]. Similarly, a neuroimaging study showed that the higher the connectivity strength of OFC, the greater the improvement of somatic symptoms, and considered that OFC played an important role in the cognitive process of pain perception [39]. There has also been a case report finding that tDCS incorporating anodic stimulation of the left DLPFC and cathodic stimulation of the right OFC can improve somatic anxiety [40]. Consequently, modulation of DLPFC and OFC could potentially alleviate somatic symptoms, in alignment with their roles in cognitive and emotional regulation. However, there is still a lack of evidence for the effectiveness of tDCS targeting DLPFC and OFC in the treatment of somatic symptoms in MDD patients, which warrants further investigation.
In summary, the therapeutic efficacy of tDCS targeting various regions in alleviating somatic symptoms in MDD patients remains ambiguous, and relevant clinical studies are lacking. Consequently, we initiated a double-blind, randomized, sham-controlled trial with the objective of comparing the efficacy of tDCS targeting DLPFC and OFC on somatic symptoms in MDD.
2 Methods
2.1 Study Design
A randomized, double-blind, sham-controlled design was used in this study. The trial was registered at the Chinese Clinical Trial Registry (ChiCTR2000034671). All participants provided written informed consent. All evaluators and tDCS therapists received consistency training in advance. According to the random sequence table prepared by the third party, participants were assigned to three research groups (OFC group, DLPFC group, and Sham group). We set up the three conditions using the built-in double-blind program of the device (double-blind “study mode”). The stimulation parameters for the sham group were the same as for the active group.
2.2 Participants
2.2.1 Inclusion and Exclusion Criteria
Right-handed patients were recruited from the outpatient clinic of Shanghai Mental Health Center from August 2020 to November 2021. Inclusion criteria were as follows: (a) patients diagnosed with major depressive episode according to the Diagnostic and Statistical Manual of Mental Disorders 5th (DSM-5) criteria and established using the Mini-International Neuropsychiatric Interview (MINI; Version 5.0.0); (b) the total score of 17-item Hamilton Depression Rating Scale (HAMD-17) ≥ 14 and the score for item 1 of the HAMD-17 ≥ 2; (c) the total score of Depression and Somatic Symptoms Scale (DSSS) ≥ 3 and the score for somatic subscale (SS) ≥ 1; (d) aged 18–65 years old; and (e) possessing the ability of listening, speaking, reading, writing, and comprehension to complete the study.
Exclusion criteria were as follows: high suicide risk as defined by psychiatrists; other psychiatric and neurological disorders; metal implants in the head; substance abuse or dependence; current treatment with benzodiazepines (as this may alter the effects of tDCS); mania and hypomania episode defined as a score < 5 on the Young Manic Rating Scale (YMRS); pregnancy; had received other neuromodulation therapy within the last 3 months.
Throughout the intervention period, participants either did not receive medication or maintained a stable antidepressant regimen and dosage, which did not change for at least 2 weeks before being included in this study.
2.2.2 Sample Size
The sample size for this pilot study was estimated based on previous studies [41, 42]. One-way analysis of variance (ANOVA) (PASS 15) was used to calculate the sample size. As a pilot study, the parameters were set as equal numbers of participants in the three groups, effect size f = 0.50, α = 0.05, power = 80%, and a dropout rate of 20%. Sample sizes of 18, 18, and 18 were obtained for each study group and 54 for the total.
2.3 tDCS Protocol
The Starstim Multichannel stimulator (Neuroelectrics, Spain) was used to perform tDCS stimulation. Sponge electrodes (5 × 5 cm) were placed over the OFC and DLPFC areas at Fp1/Fp2 or F3/F4, respectively, according to the international system of 10–20 EEG [43]. The anode was placed over F3 (left) and the cathode over F4 (right) in the DLPFC group. The anode was placed over Fp1 (left) and the cathode over Fp2 (right) in the OFC group. All three groups included a 30s ramp-up and a 30s ramp-down, but during the 20-min stimulation period, the active stimulation was 2 mA and the sham stimulation was 0 mA.
2.4 Study Procedure
Participants were scheduled to complete 10 interventions during the initial 2-week period and 1 intervention per week for the following 2 weeks based on a previous randomized controlled trial [44]. All baseline (week 0) assessments were completed prior to the initial intervention. Clinical scale assessments were conducted at the end of the 10 interventions (week 2) and at the end of maintenance treatment (week 4), and follow-up assessments were conducted at weeks 6 and 8 (compared with baseline).
2.5 Measures of Outcomes
The severity of depression was assessed with HAMD-17 [45] and Maier subscale (MS), which focuses on core depression symptoms and excludes somatic symptoms [46]. DSSS is a simple and self-administered scale with 22 items, and it has been demonstrated to have good reliability and validity for depression, with a high correlation with HAMD outcomes [47]. Compared to the conventional scales, the DSSS evaluates somatic symptoms more accurately [47, 48]. DSSS items were grouped into two domains: depression subscale (DS) and SS. Depressive and somatic symptoms were assessed with the DSSS score and SS score. The Hamilton Anxiety Rating Scale (HAMA) was used to quantify anxiety severity [49]. HAMA is a 14-item measure consisting of psychic anxiety (PA) and somatic anxiety (SA). In this study, HAMA, PA, and SA scores were used to evaluate the anxiety and somatic symptoms of anxiety.
The primary outcomes were the reduction rate of DSSS, SS and SA score from baseline to week 2 and 4. Secondary outcomes were the reduction rate of HAMD-17, MS, HAMA, and PA score at week 2 and 4. Other outcomes included the assessment of each scale during the follow-up period and predictive analysis.
2.6 Adverse Effects and Safety
Adverse effects were assessed using the tDCS Adverse Effects Questionnaire, with severity ratings ranging from 0 (none) to 3 (severe) [50]. The YMRS [51] was used to detect any possible hypomanic and manic episodes. Suicidal ideation and behaviors during the intervention period were also evaluated by experienced psychiatrists.
2.7 Statistical Analysis
Statistical analysis was performed using SPSS26.0 software (IBM). All statistical results were two-sided tests, and p < 0.05 was considered statistically significant. All data were tested for normality by Shapiro–Wilk test. GraphPad Prism 9.0.0 version was used to graph.
Differences in participant characteristics among three groups were tested using χ2 tests for categorical variables and one-way ANOVA for continuous variables. The efficacy results of the three intervention groups were analyzed by one-way ANOVA or Welch test and differences between groups were analyzed using Bonferroni or Games–Howell post hoc test, which depended on homogeneity of variance. Non-normally distributed continuous variables were compared using a nonparametric test. No data imputation was performed. The changes of scores over time in different tDCS intervention groups at each time point and the interaction effects were compared by repeated-measures ANOVA with Bonferroni post hoc test.
The general linear model and generalized linear model were used to investigate whether any predictor factors, including clinical characteristics (age at first onset, use of medication, baseline score) or demographic measures (gender, age, education, marital status, employment status), influenced tDCS outcomes. These models included factors of the group, the predictor factor, and the interaction between these factors. The correlation between the reduction rate and these predictor factors was analyzed by Pearson's correlation analysis and Spearman's correlation analysis.
3 Results
3.1 Clinical and Demographic Characteristics
A total of 155 patients met the inclusion criteria, of which 85 were excluded for various reasons, ultimately leaving 70 patients who were randomly assigned to the three groups to participate in the study. As shown in Table S1, 57 (81.4%) and 52 (74.3%) participants completed the 2-week and 4-week interventions and assessments, respectively. The numbers of individuals who completed the 6-week and 8-week follow-ups were 41 (58.6%) and 27 (38.6%). Reasons for dropping out were similar among three groups (Figure S1).
Demographics and baseline clinical characteristics of patients are listed in Table 1. Except for the score of QIDS-16SR (F(2,67) = 3.498, p = 0.036), there were no statistically significant differences among the three groups at baseline.
| OFC group (n = 23) | DLPFC group (n = 23) | Sham group (n = 24) | F/χ2 | p | |
|---|---|---|---|---|---|
| Mean (SD)/n (%) | |||||
| Gender (male) | 6 (26.1%) | 8 (34.8%) | 4 (16.7%) | 2.020 | 0.364 |
| Age (years) | 26.7 (8.5) | 25.7 (5.4) | 27.3 (5.6) | 0.326 | 0.723 |
| Education (years) | 14.7 (3.1) | 16.0 (4.0) | 15.6 (2.6) | 0.940 | 0.396 |
| Marital status | |||||
| Single | 16 (69.6%) | 15 (65.2%) | 15 (62.5%) | 4.905 | 0.768 |
| In relationship | 2 (8.7%) | 5 (21.7%) | 5 (20.9%) | ||
| Married | 4 (17.4%) | 3 (13.0%) | 4 (16.7%) | ||
| Divorced | 1 (4.3%) | 0 (0.0%) | 0 (0.0%) | ||
| Work status | |||||
| Employment | 11 (47.8%) | 10 (43.5%) | 10 (41.7%) | 5.932 | 0.821 |
| Student | 6 (26.1%) | 7 (30.4%) | 6 (25.0%) | ||
| Unemployment | 6 (26.1%) | 6 (26.1%) | 8 (33.3%) | ||
| Smoker | 7 (30.4%) | 2 (8.7%) | 6 (25.0%) | 3.505 | 0.173 |
| Age at first onset | 21.9 (7.0) | 20.9 (5.6) | 21.3 (6.7) | 0.139 | 0.870 |
| On medication | 14 (60.9%) | 7 (30.4%) | 10 (44.3%) | 4.419 | 0.110 |
| DSSS | 31.2 (11.9) | 25.3 (11.2) | 28.8 (10.3) | 1.659 | 0.198 |
| DS | 20.2 (7.0) | 17.7 (7.1) | 21.0 (6.2) | 1.513 | 0.228 |
| SS | 11.0 (6.1) | 7.6 (5.5) | 7.8 (6.5) | 2.320 | 0.106 |
| HAMD-17 | 21.7 (3.8) | 19.8 (3.4) | 21.3 (3.1) | 1.971 | 0.147 |
| MS | 10.74 (1.6) | 10.35 (2.2) | 10.75 (1.8) | 0.505 | 0.777 |
| MADRS | 27.1 (8.5) | 25.4 (7.7) | 30.5 (7.8) | 2.553 | 0.085 |
| QIDS-SR16 | 21.3 (5.7) | 17.0 (5.8) | 18.6 (4.9) | 3.498 | 0.036* |
| SSI | 15.7 (13.9) | 15.4 (12.9) | 18.0 (14.2) | 0.257 | 0.774 |
| HAMA | 16.3 (4.6) | 14.0 (5.6) | 15.0 (5.5) | 1.152 | 0.322 |
| SA | 5.2 (2.9) | 4.1 (2.9) | 3.8 (3.1) | 1.423 | 0.248 |
| PA | 11.1 (2.5) | 9.9 (3.7) | 11.2 (3.5) | 1.141 | 0.326 |
- Abbreviations: DLPFC, dorsolateral prefrontal cortex; DS, depression subscale; DSSS, Depression and Somatic Symptoms Scale; HAMA, the Hamilton Anxiety Rating Scale; HAMD-17, the 17-item Hamilton Depression Rating Scale; MADRS, Montgomery–Asberg Depression Rating Scale; MS, Maier subscale; OFC, orbitofrontal cortex; PA, psychic anxiety; QIDS-SR16, the 16-Item Quick Inventory of Depressive Symptomatology; SA, somatic anxiety; SS, somatic subscale; SSI, Beck Scale for Suicide Ideation.
- *p < 0.05.
3.2 Effect on Somatic Symptoms
The primary outcomes of tDCS are presented in Tables 2 and 3. The reduction rate of the DSSS score at week 2 after primary treatment was 35.71% ± 26.42% in the OFC group (n = 20), 52.54% ± 26.65% in the DLPFC group (n = 18), and 27.59% ± 17.45% in the Sham group (n = 19), showing a significant difference among three groups (F(2,54) = 5.224, p = 0.008). The reduction rate of the SS score was 35.34% ± 39.35% in the OFC group, 61.21% ± 40.55% in the DLPFC group, and 19.745% ± 42.27% in the Sham group, also showing a significant difference among three groups (H (df = 2) = 8.156, p = 0.017). In terms of the reduction rate of the SA score, there was no significant difference among the three groups (H (df = 2) = 4.253, p = 0.119) (Table 2).
| Group | Mean ± SD | Reduction rate (% ± SD)a | F/H | p b | |
|---|---|---|---|---|---|
| DSSS | OFC (n = 20) | 20.90 ± 11.40 | 35.71 ± 26.42 | 5.224 | 0.008** |
| DLPFC (n = 18) | 12.67 ± 10.02 | 52.54 ± 26.65 | |||
| Sham (n = 19) | 19.74 ± 9.02 | 27.59 ± 17.45 | |||
| SS | OFC (n = 20) | 7.85 ± 5.20 | 35.34 ± 39.35 | 8.156 | 0.017* |
| DLPFC (n = 18) | 3.33 ± 4.04 | 61.21 ± 40.55 | |||
| Sham (n = 19) | 5.05 ± 5.04 | 19.74 ± 42.27 | |||
| SA | OFC (n = 20) | 2.45 ± 2.31 | 62.00 ± 36.13 | 4.253 | 0.119 |
| DLPFC (n = 18) | 2.61 ± 2.00 | 17.47 ± 89.92 | |||
| Sham (n = 19) | 1.74 ± 2.18 | 31.88 ± 100.82 |
| Post hoc test | ||||
|---|---|---|---|---|
| DSSS | Bonferroni adjusted pc | SS | Bonferroni adjusted pc | |
| OFC vs. Sham | 0.881 | 0.791 | ||
| DLPFC vs. Sham | 0.007** | 0.014* | ||
| OFC vs. DLPFC | 0.104 | 0.231 | ||
- Abbreviations: DLPFC, dorsolateral prefrontal cortex; DSSS, Depression and Somatic Symptoms Scale; OFC, orbitofrontal cortex; SA, somatic anxiety; SS, somatic subscale.
- a Reduction rate, calculated as (score at baseline—score at week 2)/score at baseline) × 100.
- b p values of one-way ANOVA (DSSS) or Kruskal–Wallis test (SS and SA).
- c Adjusted p values of Bonferroni post hoc test of the reduction rate.
- *p < 0.05; **p < 0.01.
| Group | Mean ± SD | Reduction rate (% ± SD)a | p b | |
|---|---|---|---|---|
| DSSS | OFC (n = 19) | 18.11 ± 11.14 | 45.43 ± 27.22 | 0.012* |
| DLPFC (n = 17) | 7.71 ± 6.61 | 69.37 ± 21.92 | ||
| Sham (n = 16) | 13.06 ± 8.55 | 44.89 ± 38.88 | ||
| SS | OFC (n = 19) | 5.79 ± 4.96 | 52.72 ± 40.43 | 0.009** |
| DLPFC (n = 17) | 1.00 ± 1.70 | 89.06 ± 18.59 | ||
| Sham (n = 16) | 3.19 ± 3.82 | 45.96 ± 72.07 | ||
| SA | OFC (n = 19) | 3.84 ± 3.06 | 31.71 ± 46.00 | 0.170 |
| DLPFC (n = 17) | 1.82 ± 1.98 | 45.10 ± 71.42 | ||
| Sham (n = 16) | 0.94 ± 1.29 | 37.50 ± 94.62 |
| Post hoc test | ||||
|---|---|---|---|---|
| DSSS | Adjusted pc | SS | Adjusted pc | |
| OFC vs. Sham | 0.999 | 1.000 | ||
| DLPFC vs. Sham | 0.090 | 0.111 | ||
| OFC vs. DLPFC | 0.017* | 0.008** | ||
- Abbreviations: DLPFC, dorsolateral prefrontal cortex; DSSS, Depression and Somatic Symptoms Scale; OFC, orbitofrontal cortex; SA, somatic anxiety; SS, somatic subscale.
- a Reduction rate, calculated as (score at baseline—score at week 4)/score at baseline) × 100.
- b p values of Welch (DSSS) or Kruskal–Wallis test (SS and SA).
- c Adjusted p values of Games–Howell (DSSS) and Bonferroni (SS and SA) post hoc test of the reduction rate.
- *p < 0.05; **p < 0.01.
Post hoc analysis showed that at week 2, the reduction rate for DSSS was higher in the DLPFC group compared with the Sham group (adjusted p = 0.007), and the reduction rate for SS was also significantly different between DLPFC group and Sham group (adjusted p = 0.014) (Table 2).
After the maintenance treatment, the DSSS score at week 4 decreased 45.43% ± 27.22% in the OFC group (n = 19), 69.37% ± 21.92% in the DLPFC group (n = 17), and 44.89% ± 38.88% in the Sham group (n = 16). The reduction rate for SS was 52.72% ± 40.43% in the OFC group, 89.06% ± 18.59% in the DLPFC group, and 45.96% ± 72.07% in the Sham group. Significant differences were observed in the reduction rates for both DSSS (F(2,30.546) = 5.087, p = 0.012) and SS (H (df = 2) = 9.432, p = 0.009) among three groups at week 4. No significant difference was found among groups for the SA score reduction rates (H (df = 2) = 3.549, p = 0.170) (Table 3).
Post hoc multiple comparison at week 4 revealed that the DLPFC group outperformed the OFC group in both DSSS (adjusted p = 0.017) and SS (adjusted p = 0.008) score reduction rates, although there was no significant difference compared to the Sham group (Table 3).
The reduction rate of the scale score for somatic symptoms is shown in Figure 1.
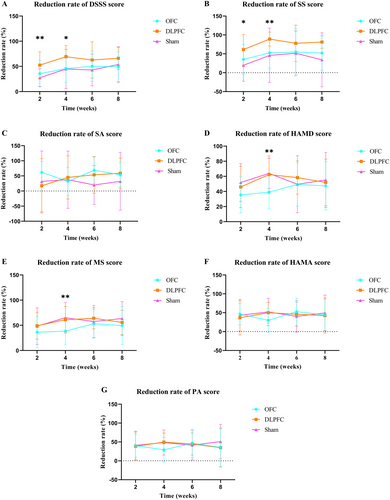
3.3 Effect on Depression and Anxiety
HAMD-17 and MS scores were reduced in all three groups after the 2-week and 4-week interventions. However, there was no significant difference in the reduction rates at week 2 among three groups (HAMD-17: F(2,54) = 2.120, p = 0.130; MS: F(2,54) = 1.010, p = 0.371) (Table 4).
| Group | Mean ± SD | Reduction rate (% ± SD)a | F/H | p b | |
|---|---|---|---|---|---|
| HAMD-17 | OFC (n = 20) | 14.30 ± 5.85 | 35.43 ± 23.91 | 2.120 | 0.130 |
| DLPFC (n = 18) | 10.33 ± 5.02 | 45.96 ± 27.22 | |||
| Sham (n = 19) | 10.26 ± 5.91 | 51.91 ± 24.98 | |||
| MS | OFC (n = 20) | 6.90 ± 3.35 | 36.27 ± 30.95 | 1.010 | 0.371 |
| DLPFC (n = 18) | 5.28 ± 2.65 | 49.07 ± 26.57 | |||
| Sham (n = 19) | 5.37 ± 3.47 | 48.40 ± 36.33 | |||
| HAMA | OFC (n = 20) | 8.90 ± 5.21 | 46.37 ± 28.37 | 0.336 | 0.845 |
| DLPFC (n = 18) | 8.67 ± 5.67 | 36.55 ± 45.49 | |||
| Sham (n = 19) | 7.84 ± 5.56 | 43.32 ± 41.78 | |||
| PA | OFC (n = 20) | 6.45 ± 3.43 | 40.48 ± 30.96 | 0.025 | 0.976 |
| DLPFC (n = 18) | 6.06 ± 3.83 | 38.52 ± 37.81 | |||
| Sham (n = 19) | 6.11 ± 3.83 | 40.99 ± 37.72 |
- Abbreviations: DLPFC, dorsolateral prefrontal cortex; HAMA, the Hamilton Anxiety Rating Scale; HAMD-17, the 17-item Hamilton Depression Rating Scale; MS, Maier subscale; OFC, orbitofrontal cortex; PA, psychic anxiety.
- a Reduction rate, calculated as (score at baseline—score at week 2)/score at baseline) × 100.
- b p values of one-way ANOVA (HAMD-17, MS, and PA) or Kruskal–Wallis test (HAMA).
The difference in the reduction rates among the three groups was statistically significant after maintenance treatment at week 4 (HAMD-17: H (df = 2) = 12.511, p = 0.002; MS: F(2,49) = 4.738, p = 0.013). Post hoc multiple comparisons showed that the reduction rate of HAMD-17 score in the OFC group was lower than that in the Sham group (adjusted p = 0.009) and the DLPFC group (adjusted p = 0.006). And the reduction rate of MS score in the OFC group was only significantly lower than that in the Sham group (adjusted p = 0.022) (Table 5).
| Group | Mean ± SD | Reduction rate (% ± SD)a | F/H | p b | |
|---|---|---|---|---|---|
| HAMD-17 | OFC (n = 19) | 13.53 ± 5.18 | 39.10 ± 22.21 | 12.511 | 0.002** |
| DLPFC (n = 17) | 7.00 ± 3.95 | 62.38 ± 24.83 | |||
| Sham (n = 16) | 7.56 ± 4.38 | 63.97 ± 19.93 | |||
| MS | OFC (n = 19) | 6.68 ± 2.79 | 38.59 ± 27.12 | 4.738 | 0.013* |
| DLPFC (n = 17) | 3.88 ± 2.32 | 61.17 ± 26.25 | |||
| Sham (n = 16) | 3.63 ± 2.80 | 65.04 ± 30.18 | |||
| HAMA | OFC (n = 19) | 11.68 ± 6.34 | 29.82 ± 31.81 | 2.677 | 0.079 |
| DLPFC (n = 17) | 6.65 ± 4.36 | 50.11 ± 27.53 | |||
| Sham (n = 16) | 6.00 ± 3.76 | 52.02 ± 36.13 | |||
| PA | OFC (n = 19) | 7.84 ± 4.00 | 29.08 ± 32.81 | 2.613 | 0.084 |
| DLPFC (n = 17) | 4.82 ± 2.79 | 49.58 ± 23.77 | |||
| Sham (n = 16) | 5.06 ± 2.67 | 48.36 ± 33.19 |
| Post hoc test | ||||
|---|---|---|---|---|
| HAMD-17 | Adjusted pc | MS | Adjusted pc | |
| OFC vs. Sham | 0.009** | 0.022* | ||
| DLPFC vs. Sham | 1.000 | 1.000 | ||
| OFC vs. DLPFC | 0.006** | 0.056 | ||
- Abbreviations: DLPFC, dorsolateral prefrontal cortex; HAMA, the Hamilton Anxiety Rating Scale; HAMD-17, the 17-item Hamilton Depression Rating Scale; MS, Maier subscale; OFC, orbitofrontal cortex; PA, psychic anxiety.
- a Reduction rate, calculated as (score at baseline—score at week 4)/score at baseline) × 100.
- b p values of one-way ANOVA (HAMA, MS, and PA) or Kruskal–Wallis test (HAMD-17).
- c Adjusted p values of Bonferroni post hoc test of the reduction rate.
- *p < 0.05; **p < 0.01.
All three groups exhibited a decrease in anxiety, as measured by HAMA and PA, although there was no significant difference in the reduction rates among the three groups at week 2 (HAMA: H (df = 2) = 0.336, p = 0.845; PA: F(2,54) = 0.025, p = 0.976) (Table 4) and week 4 (HAMS: F(2,49) = 2.677, p = 0.079; PA: F(2,49) = 2.613, p = 0.084) (Table 5).
The reduction rate of depression and anxiety scale score is shown in Figure 1.
3.4 Assessment of 6- and 8-Week Follow-Ups
At the 6- and 8-week follow-ups (Table S2), DSSS, SS, and SA scores were significant on the session's main effect, suggesting that scores reduced significantly from baseline to each time point. The group's main effect was significant on the 6-week DSSS score (p = 0.035) and the 6-week (p = 0.019) and 8-week (p = 0.011) SS scores. None of the session × group interaction effects for DSSS and SS scores were significant, while SA scores showed significance (p = 0.013) on the 6-week session × group interaction effect, suggesting the change in SA scores varied across the three groups over time progressed (Figure S2C). Post hoc analysis suggested that both DSSS (p = 0.035) and SS (p = 0.017) scores showed a greater decrease in the DLPFC group than in the OFC group at week 6 but were not significantly different from the Sham group (Figure S2A,B). At week 8, there was a lower decrease in SS scores in the OFC group compared to the DLPFC group (p = 0.020).
For HAMD-17 scores at 6- and 8-week follow-up (Table S2), significant differences were observed for the session main effects at week 6 (p < 0.001) and week 8 (p < 0.001), as well as for the group main effect at week 6 (p = 0.009), whereas no significant differences were observed for the session × group interaction effect. Post hoc analysis of the main effect of the group at week 6 showed that compared to OFC group, the DLPFC group (p = 0.011) showed more decrease in HAMD-17 scale scores (Figure S2D).
In addition to significance on the session's main effect, HAMA (p = 0.044) showed significance on the 6-week session × group interaction effect, indicating that by week 6, the magnitude of change in HAMA scores differed among the three groups as time progressed and the DLPFC group showed a better improvement than the OFC group (Figure S2E). No group's main effect showed significant difference (Table S2).
3.5 Predictive Factor Analysis
Analysis of clinical and demographic factors affecting depressive and somatic symptoms (Table S3) showed that the reduction rate of DSSS score (after 10 Interventions) was negatively associated with gender as female, SSI score, DSSS score, and SS score at baseline, regardless of the group. It also revealed a significant interaction (p = 0.021) between age and tDCS group, and further analysis showed that in the OFC group, older participants showed a higher DSSS score reduction rate. The significant interaction (p = 0.011) between single or not and group showed that participants in a relationship performed better than the single individuals in the DLPFC group.
Correlation analysis of clinical demographic data with improvement in anxiety symptoms found a positive correlation between age and the rate of HAMA score reduction (after 10 Interventions) (Table S4). Also, general linear model results showed that the higher the HAMA score at baseline, the higher the rate of HAMA score reduction after 10 interventions (p = 0.032), regardless of group.
3.6 Safety Outcomes
As shown in Table S5, all the adverse effects item ratings showed no differences among the three groups. Meanwhile, no patients had manic episodes during the intervention and follow-up, with YMRS scores of ≤ 5.
4 Discussion
This double-blind, randomized, sham-controlled clinical study compared the efficacy of tDCS targeting DLPFC and OFC on somatic symptoms in patients with MDD. After 2 weeks of primary treatment, the DLPFC group demonstrated a more pronounced improvement in depressive somatic symptoms compared to the Sham group. At week 4 and follow-up periods, the DLPFC group outperformed the Sham and OFC groups, but did not show a significant difference with the Sham group. No significant improvement in depression and anxiety symptoms was observed in the active groups compared to the Sham group. The tDCS interventions were well tolerated, with no major adverse events reported.
4.1 Effect on Somatic Symptoms
The research discovered that anodic stimulation of the left DLPFC demonstrated superior effectiveness in alleviating depressive somatic symptoms, whereas cathodic stimulation of the right OFC appeared ineffectual. There was no difference in improvement of core depressive symptoms among the three groups in terms of MS score, which excludes somatic symptoms, further confirming the efficacy of DLPFC in treating somatic symptoms in MDD. This implies the possibility of alleviating depressive somatic symptoms through activation of the left DLPFC. The DLPFC is a cognitive-executive-control brain region processing expectancy where cognitive modulations of pain may initiate, then trigger the descending pain modulation system and reward system to diminish or intensify one's pain experience depending on context [52]. Given the paucity of studies exploring the therapeutic influence of tDCS on somatic symptoms in depressive patients, this study's findings align with previous research on pain and sleep disorders, suggesting that anodic stimulation of the left DLPFC via tDCS might present a potent approach to ameliorate depressive somatic symptoms [36, 37].
In our research, it was observed that tDCS targeting OFC did not exhibit superior effectiveness compared to the Sham group. Neuroimaging research indicated a correlation between chronic pain and sustained hypofunction in OFC [53]. Moreover, an interesting correlation was discovered that the stronger the connectivity of the OFC, the more pronounced the reduction in pain intensity [39]. Therefore, anodal stimulation of the OFC to enhance its activity without cathodic stimulation of right OFC may contribute to alleviating somatic symptoms. Previous studies also found that anodal stimulation targeting the primary motor cortex can alleviate fatigue and pain, implying that the primary motor cortex could be another effective target for alleviating somatic symptoms in patients with MDD [54, 55].
Post-treatment follow-up revealed that tDCS sustained its impact on depressive somatic symptoms for up to a month, although with no significant efficacy difference between the DLPFC group and the sham group, which is consistent with the prolonged effect on depressive symptoms [26]. Intensified electrical stimulation, including a stronger current and twice-daily sessions, has been shown to be more effective in other psychiatric disorders [56, 57]. Intensified stimulation may also have a stronger effect in patients with MDD. Further research with a larger sample size is imperative to better interpret the effects on depressive somatic symptoms of tDCS targeting different regions and underlying mechanisms.
Concerning somatic anxiety, neither the DLPFC nor the OFC groups showed any significant effect. However, at the 6-week follow-up, the DLPFC group appeared to outperform the OFC group, although this does not constitute definitive proof of its efficacy. While DLPFC and OFC have been linked to somatic anxiety, clinical research in this area remains scant. Only a single case report exists to support the effect of tDCS on somatic anxiety [40]. Thus, which brain region tDCS is effective in relieving somatic anxiety by stimulating is still unknown. Further exploration is required to identify a suitable tDCS treatment paradigm.
4.2 Efficacy on Depression Severity
Neither OFC-tDCS nor DLPFC-tDCS demonstrated superiority over the Sham group in terms of depressive symptoms. In fact, the OFC group's performance was inferior to that of the DLPFC group and Sham group in terms of HAMD-17 scores. Several factors may elucidate the finding. First, the optimal electrode positioning for OFC-tDCS in depression treatment remains undetermined, which may have influenced the results of this study. In light of prior research indicating that inhibitory stimulation of the right OFC (FP2) could potentially alleviate depression symptoms, the decision was made to place the cathode over the OFC (Fp2). Nevertheless, these findings were derived from self-reported measures and lacked clinician evaluations and sham controls, and they were not conducted within a depression cohort [32, 33].
Second, a recent TMS-EEG analysis identified that OFC activity in depressed patients was lower than that in healthy controls prior to treatment. However, following active rTMS treatment, OFC activity significantly increased in depressed patients. This suggests that activating the OFC could potentially mitigate depression symptoms. Contrarily, our study inhibited the right-sided OFC, which may have diminished the antidepressant effect of tDCS [58]. In addition, similar to our study, a previous study found no difference in antidepressant efficacy between tDCS anodic stimulation of DLPFC and the Sham group, which assumed that low currents delivered under the Sham tDCS condition were biologically active and could not be discounted [23].
Moreover, medications taken during tDCS treatment may also have an impact on the effectiveness of the treatment [59]. Medications that affect different neurotransmitter systems such as GABA, dopamine and 5-hydroxytryptophan can all affect the effect of tDCS on tissue excitability [60]. Finally, our pilot study may have been insufficient in detecting differences. A study with a larger sample size could potentially uncover more pronounced differences and expanding the scope of the study would contribute to a more comprehensive understanding of these findings. It may be possible to improve the efficacy of tDCS in the treatment of depression by optimizing the therapeutic target, refining the current intensity, and controlling the clinical characteristics of the participants (e.g., minimizing the use of antidepressants), among other things.
4.3 Other Results
Our study did not find a significant improvement of tDCS treating anxiety symptoms of patients with MDD. A review of the effects of tDCS on anxiety in different samples showed some positive effects, but differences in the design and measures used did not provide conclusive evidence of its effectiveness for specific anxiety disorders or anxiety in depression [61]. The focus of this study was not on anxiety symptoms, but it is also worth further exploration.
The study assessed the predictive value of age and relationship status in response to tDCS intervention. Findings indicated that older participants in the OFC group and participants in a relationship in the DLPFC group experienced a more pronounced alleviation of depression and somatic symptoms. Furthermore, more severe baseline anxiety severity corresponded to a greater rate of HAMA score reduction. However, given the limited sample size of this preliminary study, it is necessary to conduct curative effect prediction research in a larger population in the future.
4.4 Limitations
There are several limitations that need to be mentioned in this study. Firstly, the restricted sample size and the predominance of online follow-ups, a measure necessitated by the COVID-19 pandemic, may have influenced the results. Secondly, some participants concurrently received antidepressants during the tDCS intervention, which is more practical in a clinical setting. The combined impact of tDCS and medication could potentially alter treatment efficacy [59, 60]. However, participants were required to maintain a consistent medication regimen for a minimum of 2 weeks before and throughout the 10 interventions. Moreover, further analysis revealed no significant correlation between medication use and effectiveness. Therefore, this factor is unlikely to have significantly affected the study outcomes. Finally, this study is a preliminary study focusing on somatic symptoms in patients with MDD. In further studies, parallel controlled trials should be carried out, combined with physiological measurement and cognitive evaluation, to investigate the efficacy of different targets of tDCS in MDD patients with different severity of somatic symptoms.
5 Conclusion
In conclusion, the tDCS treatment targeting DLPFC proved to be more effective in ameliorating depressive somatic symptoms than the sham procedure and appeared to surpass the stimulation of OFC. No significant improvement in the severity of anxiety and depression was observed in active groups compared to the sham group. Our study suggested the tDCS targeting DLPFC may be a potentially effective therapeutic target for alleviating somatic symptoms in patients with MDD.
Acknowledgments
This work was supported by the Shanghai “Science and Technology Innovation Action Plan” Medical Innovation Research (21Y11905600); Shanghai “Science and Technology Innovation Action Plan” Natural Science Foundation of Shanghai (21ZR1455100); Science and Technology Innovation Project of Shanghai Jiao Tong University School of Medicine (Humanities and Social Sciences) (No. WK2017); STI2030-Major Projects (2021ZD0200800); National Key R&D Program of China (2022YFC3302001); and Research Physician Program of Shanghai Mental Health Center (2021-YJXYS-06).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




