Unveiling the interoception impairment in various major depressive disorder stages
Abstract
Background
The intricate pathophysiological mechanisms of major depressive disorder (MDD) necessitate the development of comprehensive early indicators that reflect the complex interplay of emotional, physical, and cognitive factors. Despite its potential to fulfill these criteria, interoception remains underexplored in MDD. This study aimed to evaluate the potential of interoception in transforming MDD's clinical practices by examining interoception deficits across various MDD stages and analyzing their complex associations with the spectrum of depressive symptoms.
Methods
This study included 431 healthy individuals, 206 subclinical depression individuals, and 483 MDD patients. Depressive symptoms and interoception function were assessed using the PHQ-9 and MAIA-2, respectively.
Results
Interoception dysfunction occurred in the preclinical phase of MDD and further impaired in the clinical stage. Antidepressant therapies showed limited efficacy in improving interoception and might damage some dimensions. Interoceptive dimensions might predict depressive symptoms, primarily enhancing negative thinking patterns. The predictive model based on interoception was built with random split verification and demonstrated good discrimination and predictive performance in identifying MDD.
Conclusions
Early alterations in the preclinical stage, multivariate associations with depressive symptoms, and good discrimination and predictive performance highlight the importance of interoception in MDD management, pointing to a paradigm shift in diagnostic and therapeutic approaches.
1 BACKGROUND
Major depressive disorder (MDD), as a prevalent affective disorder, has experienced a substantial rise in incidence worldwide, becoming a significant global challenge. However, the current diagnostic and treatment protocols for MDD are suboptimal. Primarily, MDD often begins insidiously, presenting initially as vague neurovegetative symptoms such as fatigue, sleeplessness, or unexplained pain,1, 2 which are frequently interpreted as signs of other medical conditions, causing delays in diagnosis and treatment. Furthermore, the symptom heterogeneity of MDD complicates the accuracy of diagnosis and the understanding its pathophysiological mechanisms. Despite the “Three-Dimensional Symptom Model” categorizing MDD symptoms into emotional, physical, and cognitive domains as a means to understand MDD's heterogeneity, it primarily emphasizes symptomatic manifestations, neglecting the underlying biological mechanisms.3 The subjective nature and clinical overlap of isolated symptoms also limit the efficacy of symptom-based neurobiological mappings in MDD research.4 Finally, the variability in individual responses to antidepressant treatment often leads to persistent residual symptoms like sleep disturbances or fatigue,5 adversely affecting patients, quality of life and increasing the risk of recurrence.6 Therefore, developing early indicators that integrate emotional, physical, and cognitive dimensions while considering the interplay of physiological, psychological, and environmental factors is crucial for enhancing the diagnosis and treatment outcomes of MDD.
Interoception refers to the perception of the body's internal physiological state, encompassing the interpretation, integration, and regulation of these physiological signals.7 Accurate interoceptive perception is essential for homeostatic balance, yet deviations in this process can lead to an exaggerated focus on or misinterpretation of these signals, influencing self-perception and identity.8 Such aberrant interoception may trigger negative emotions like anxiety, tension, and fear, and induce unhealthy physiological responses that lead individuals to adopt maladaptive psychological coping strategies,9 making interoception impairment a potential risk factor for psychiatric conditions.10
Moreover, the insula, central to the generation and regulation of interoception,11 is intricately linked to somatic perception, emotional processing, cognitive functions, and autonomic regulation.12-14 Therefore, interoception is often explored to explain the emotional, somatic, and cognitive symptoms of various mental and somatic diseases.15 Lastly, interoception is influenced by multiple factors, including physiological states, life experiences, and health status, exhibiting strong individualized characteristics.16 These features make interoception a promising area to meet the diagnostic and therapeutic needs of MDD. Recent research indicates a potential link between MDD and interoception, with studies showing reduced cardiac interoceptive responses and diminished insular activation during cardiac interoception tasks in MDD patients.15-20 Previous research has indicated the neural mechanisms underlying interoception and its relationship with peripheral somatic changes in depression include multiple pathways, interactions with individual differences in interoception, and a developmental psychobiological systems perspective.15, 21 A Meta-analysis further identified the disrupted dorsal mid-insula activation as a key neural region in interoception impairment across various psychiatric disorders, including MDD. Notably, mid-insula cluster differed anatomically from brain regions involved in affective processing and from regions altered by psychological or pharmacological interventions for affective disorders, suggesting interoception as an underexplored potential target for intervention.22 Moreover, the interoception neural circuitry, centered around the insula, had been proven to couple with peripheral physiological responses such as systemic inflammatory reactions and glucose metabolism,23, 24 contributing to depression-related symptoms like appetite changes,25 suicidal and non-suicidal self-injurious thoughts and behaviors,26 and sleep disturbances.27 Researches indicated that improvements in interoception can independently predict positive treatment responses,28-30 whereas a pre-treatment decline in bodily signal perception may signal a risk for persistent fatigue symptoms post-treatment.30 The current antidepressants appear to exacerbate abnormalities in bodily sensations within emotional contexts. Enhancing bodily awareness might be an important therapeutic target for MDDs.31 These all highlight the value of interoception on MDD.
However, interoception is not limited to cardiac perception but is a multidimensional, multistage psychophysiological process. It shows significant dynamic characteristics, individual differences and sensitivity to antidepressant treatment.32 Studies in this area have been limited by small sample sizes and a lack of comprehensive analysis of interoception's multidimensional characteristics across various stages of MDD, hindering the identification of specific clinical questions that interoception may address. They also insufficiently investigated the multivariate relationship between interoception and depressive symptoms, limiting insights into interoception's potential to explain the symptom heterogeneity in MDD. Furthermore, the results of these interoception studies were not internally validated, and there was a distinct lack of evaluation concerning its capacity to discriminate and predict MDD, as well as a clear deficiency in assessing its practical clinical utility. These gaps are necessary steps to validate mental health indicators and their capacity to transform clinical practices.33
Therefore, this study aimed to explore the variations in interoception across different stages of MDD, including subclinical and medication-naïve phases, and how these variations correlate with depressive symptoms. The discrimination and predictive performance of interoception in MDD were also evaluated. Addressing these gaps would clarify the role of interoception in MDD and hold significant theoretical and practical implications for optimizing the diagnosis and treatment of depression. We hypothesized that (1) all dimensions of interoception are impaired in individuals with MDD, evident even in the subclinical phase; (2) interoception primarily contributes to the MDD through affecting somatic symptoms; and (3) measuring multidimensional interoception can help identify MDD, potentially enhancing clinical outcomes.
2 MATERIALS AND METHODS
Ethics approval was provided by The Affiliated Mental Health Center of Jiangnan University Ethics Boards (Ethical approval number: WXMHCIRB2021LLky115). All participants provided written informed consent and were compensated 40.00 Chinese Yuan.
2.1 Subject recruitment and assessment
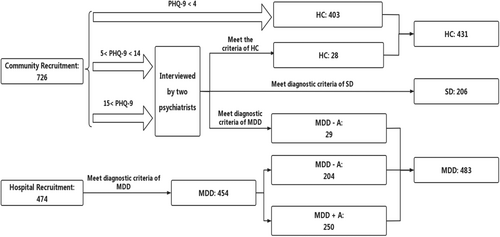
A total of 1120 participants (healthy controls [HCs]: 431, subthreshold depression patients [SDs]: 206, MDD patients [MDDs]: 483) were recruited by advertisement in the community and Wuxi Mental Health Center from May 2022 to September 2023.
The inclusions criteria for HC group were (1) age range from 18 to 65 years old; (2) not meeting the criteria for any Diagnostic and Statistical Manual 5th Edition (DSM-5) axis I disorder or personality disorders, as assessed by the Structured Clinical Interview for DSM-5; (3) no history of any kind of mental disorder; and (4) no physical illness. The exclusion criteria were (1) pregnant or perinatal women and (2) incomplete assessments.
The inclusions criteria for SD group were (1) age range from 18 to 65 years old; (2) a score of the Patient Health Questionnaire-9 (PHQ-9) between 5 and 934; and (3) with a symptom of either depressed mood or loss of interest or pleasure; (4) without any antidepressive therapy. The exclusion criteria were (1) who had suicidal ideation and suicidal attempts; (2) that the duration of symptom were above 2 weeks before enrollment; (3) who received psychotherapy (mindfulness-based cognitive therapy, etc.) and physical therapy (repetitive transcranial magnetic stimulation, etc.); and (4) returning incomplete assessments.
The inclusions criteria for MDD group were (1) age range from 18 to 65 years old; (2) meeting the MDD criteria for DSM-5; and (3) that antidepressive therapy duration were no less than 28 days. The exclusion criteria were (1) meeting the criteria for any DSM-5 axis I disorder or personality disorders, as assessed by the Structured Clinical Interview for DSM-5; (2) who had a history of any kind of mental disorder; (3) who had any physical illness; (4) who received psychotherapy (mindfulness-based cognitive therapy, etc.) and physical therapy (repetitive transcranial magnetic stimulation, etc.); and (5) returning incomplete assessments.
All participants were assessed by two psychiatrists. Although there is still no consensus on the definition of SD,35 researchers have identified SD as an early stage or precursor of depression.36 In this study, SDs were screened using the PHQ-9 among individuals who self-identified as HCs. These individuals denied being in a depressive state, perceiving their condition merely as “recoverable stress”. All SDs were re-examined by a psychiatrist after an initial outpatient assessment. Only those who showed subthreshold depressive symptoms in assessments by two rounds of psychiatrist were included in the SD group (Figure 1).
2.2 Measures
The PHQ-9 and the Generalized Anxiety Disorder-7 questionnaire (GAD-7) were used to assess the severity of depression and anxiety.37 Multidimensional Assessment of Interoceptive Awareness-version 2 (MAIA-2) was applied to assess self-reported interoception.38 The Physical Exercise Rating Scale (PARS-3) was used to assess the amount of exercise.39 Detailed descriptions of these measures are provided in Appendix S1.
2.3 Statistical analysis
The statistical analysis consisted of three parts. First, we investigated the changes in interoception at different stages of MDD. The focus was on the pre-clinical and medication-naive populations. The groups' differences in demographic information were obtained using the χ2 test for categorical variables and the analysis of variance (ANOVA) for continuous variables. Analysis of covariance (ANCOVA) was used to compare interoceptive dimensions across subjects, controlling for a comprehensive set of demographic and lifestyle variables. These covariates included gender, age, body mass index (BMI), years of education, daily exercise duration, time spent on social media, tobacco and alcohol use, coffee and tea consumption, marital status, childbearing status, and total annual household income. Internal consistency reliability for each scale was determined using McDonald's omega or Cronbach's alpha. Post hoc comparisons were corrected using the Bonferroni method. We first compared the differences among three groups. Subsequently, within the depression group, they further subdivided participants into first-episode, medication-naïve and previously treated subgroups to analyze the effects of medication. This can effectively reduce the number of comparisons, thereby lowering the Type I error rate (detailed in Appendix S1).
Second, we examined the multivariate relationship between the interoception predictor set and the depression outcome set and determined specific variables within each set that contribute to the strength of this relationship by the Canonical Correlation Analysis (CCA).40 The CCA is a statistical method used to explore the relationships between two multivariate sets of variables. It considers multiple variables simultaneously and allows for a more holistic understanding of the relationships between two sets of variables.41
In the current study, CCA was applied to evaluate a multivariate relationship between a set of interoception predictors and a set of depression outcomes. Under this framework, we defined the interoception predictor set to consist of eight interoception subscales, and the depression outcomes set to consist of nine depressive symptoms items in PHQ-9 (Figure 2). Depressive symptoms were divided into emotional symptoms, autonomic symptoms, and neurocognitive symptoms according to previous research.3

Finally, we assessed the diagnostic efficacy of interoception for MDD. A clinical prediction model for MDD based on interoception was built using logistic regression analysis. Additionally, a nomogram will be developed based on the logistic regression model. A nomogram is a graphical representation of the logistic regression equation, allowing for the estimation of individual probabilities of depression occurrence based on the values of the predictive factors.42, 43 The performance of the nomogram was assessed using the receiver operating characteristic (ROC) curve and calibration curve. Decision Curve Analysis (DCA) was also used to evaluate the clinical utility of predictive model (Appendix S2).
The results with a p-value of <0.05 were considered statistically significant. Following the analytical steps recommended by a previous study,33 we hoped to provide evidence that interoception had the potential ability to transform MDD's clinical practices. The nomogram was developed using the “rms” package. ROC curves and DCA were constructed using the “pROC” and “riskRegression” packages, respectively. All statistical analyses were performed using the R software (version 4.2.2), along with MSTATA software (www.mstata.com).
3 RESULTS
3.1 Demographic characteristics
Table 1 presents demographic information. MDDs had fewer years of education (F = 91.868, p = 0.000) and BMI (F = 4.898, p = 0.008) than HCs. More MDDs were female and had the habits of smoking and drinking, and spent more time on social media (all p < 0.05). Other demographic information had no difference (all p > 0.05).
| Characteristics | Participants | |||
|---|---|---|---|---|
| HC (n = 431a) | SD (n = 206a) | MDD (n = 483a) | p-Valueb | |
| Age, mean (SD), years | 28 (9) | 26 (8) | 27 (10) | 0.207 |
| Gender, female (%) | 262 (61%) | 132 (64%) | 345 (71%) | 0.003 |
| Education, mean (SD), years | 15.8 (3.1) | 15.6 (2.9) | 13.3 (3.0) | <0.001 |
| Body mass index, mean (SD) | 22.5 (3.3) | 21.7 (2.9) | 21.9 (3.3) | 0.008 |
| Physical exercise, mean (SD), h/day | 12 (14) | 11 (13) | 10 (15) | 0.268 |
| Marital status (%) | ||||
| Married | 122 (28%) | 48 (23%) | 146 (30%) | 0.140 |
| Single | 301 (70%) | 153 (74%) | 319 (66%) | |
| Divorced | 8 (2%) | 5 (2%) | 18 (4%) | |
| Pregnancy, number (%) | ||||
| 0 | 388 (90%) | 178 (86%) | 377 (78%) | <0.001 |
| 1 | 29 (7%) | 20 (10%) | 85 (18%) | |
| 2 | 13 (3%) | 6 (3%) | 19 (4%) | |
| 3 | 1 (0) | 2 (1%) | 2 (0) | |
| Frequency for cigarette smoking in the past month (%) | ||||
| No smoking | 388 (90%) | 185 (90%) | 400 (83%) | 0.007 |
| >7 days | 6 (1%) | 8 (4%) | 16 (3%) | |
| 2–7 days | 19 (4%) | 4 (2%) | 34 (7%) | |
| Everyday | 18 (4%) | 9 (4%) | 33 (7%) | |
| Frequency for coffee and tea intake in the past month (%) | ||||
| No coffee and tea intake | 263 (61%) | 131 (64%) | 317 (66%) | 0.310 |
| >7 days | 96 (22%) | 50 (24%) | 103 (21%) | |
| 2–7 days | 43 (10%) | 19 (9%) | 44 (9%) | |
| Everyday | 29 (7%) | 6 (3%) | 19 (4%) | |
| Frequency for alcohol drinkers in the past month (%) | ||||
| No drinking | 382 (89%) | 182 (88%) | 414 (86%) | 0.013 |
| >7 days | 39 (9%) | 24 (12%) | 51 (11%) | |
| 2–7 days | 7 (2%) | 0 (0%) | 4 (1%) | |
| Everyday | 3 (1%) | 0 (0%) | 14 (3%) | |
| Daily social media usage time, hours (%) | ||||
| <3 | 80 (19%) | 28 (14%) | 86 (18%) | 0.004 |
| 3–5 | 160 (37%) | 87 (42%) | 154 (32%) | |
| 5–8 | 117 (27%) | 53 (26%) | 113 (23%) | |
| >8 | 74 (17%) | 38 (18%) | 130 (27%) | |
| Economic status (annual family income), Chinese Yuan (%) | ||||
| <10,000 | 191 (44%) | 84 (41%) | 122 (25%) | <0.001 |
| 10,000–30,000 | 205 (48%) | 108 (52%) | 299 (62%) | |
| 30,000–50,000 | 26 (6%) | 8 (4%) | 45 (9%) | |
| >50,000 | 9 (2%) | 6 (3%) | 17 (4%) | |
| Noticing, mean (SD) | 2.35 (1.18) | 2.58 (1.05) | 2.74 (1.03) | <0.001 |
| Not distracting, mean (SD) | 3.09 (1.10) | 2.84 (0.97) | 2.83 (1.04) | <0.001 |
| Not worrying, mean (SD) | 2.65 (0.75) | 2.40 (0.72) | 2.05 (0.93) | <0.001 |
| Attention regulation, mean (SD) | 2.23 (1.10) | 2.30 (0.85) | 2.00 (0.93) | <0.001 |
| Emotional awareness, mean (SD) | 2.35 (1.29) | 2.52 (1.01) | 2.44 (1.12) | 0.224 |
| Self-regulation, mean (SD) | 2.24 (1.22) | 2.02 (1.02) | 1.31 (0.96) | <0.001 |
| Body listening, mean (SD) | 1.99 (1.29) | 1.97 (1.00) | 1.54 (1.12) | <0.001 |
| Trusting, mean (SD) | 2.52 (1.30) | 2.29 (1.00) | 1.65 (0.98) | <0.001 |
| PHQ9, mean (SD) | 1 (1) | 7 (1) | 19 (5) | <0.001 |
| GAD7, mean (SD) | 1.0 (1.9) | 6.2 (3.6) | 13.1 (4.8) | <0.001 |
| Disease duration, mean (SD), months | — | — | 31 (45) | — |
- Abbreviation: SD, standard deviation.
- a n (%).
- b One-way ANOVA; Pearson's Chi-squared test; Fisher's exact test.
3.2 Interoception in MDD
3.2.1 Interoception changes in different stages of MDD
Adjusting for all demographic variables, our analysis revealed a trended alteration in interoception from HCs to SDs, and further to MDDs. Although SDs demonstrated increased “noticing” alongside decreased “self-regulation” and “trusting” compared with HCs, these differences did not reach statistical significance. In contrast, among MDDs, notable interoception disparities emerged, with “noticing” (F = 11.78, p < 0.001, η2 = 0.021), “self-regulation” (F = 59.68, p < 0.001, η2 = 0.097), and “trusting” (F = 44.35, p < 0.001, η2 = 0.074) significantly diverging from HCs. Moreover, MDDs exhibited significant reductions in “attention regulation” (F = 4.68, p = 0.009, η2 = 0.008) and “body listening” (F = 12.032, p < 0.001, η2 = 0.021) compared with both SDs and HCs. SDs and MDDs, who did not differ significantly from each other, performed markedly worse in “not distracting” than HCs (F = 10.56, p < 0.001, η2 = 0.019). A gradual decline in “not worrying” was observed from HCs to SDs to MDDs (F = 44.28, p < 0.001, η2 = 0.074). However, we did found the difference in “emotional awareness” (F = 2.00, p = 0.136, η2 = 0.004) (Figure 3A,C).
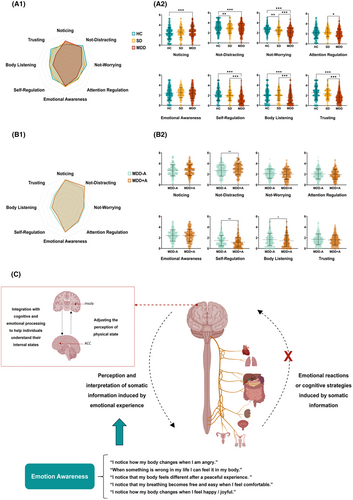
3.2.2 Effects of antidepressive therapy on interoception in MDDs
We further grouped the MDDs by with/without antidepressive therapy. There were 250 MDDs with antidepressive therapy (MDD + A) and 233 MDDs without antidepressive therapy (MDD − A). For MDDs, the antidepressive therapy duration was 36 days (Standard deviation [SD] [6]). According to the previous studies,44, 45 the mean Fluoxetine-equivalent dose of antidepressants was 37.2 (SD [1.2]) mg/day, and the mean chlorpromazine-equivalent dose of antipsychotics was 348.6 (SD [95.1]) mg/day. The demographics and baseline characteristics of MDD − A and MDD + A, and antidepressive agents for MDD + A are listed in Appendix S3.
After controlling for all demographic characteristics, disease duration, and anxiety, MDD + A showed improved “not distracting” (F = 9.36, p = 0.002, η2 = 0.020), and further exacerbated “self-regulation” (F = 8.57, p = 0.004, η2 < 0.001) and “body listening” (F = 6.03, p = 0.014, η2 = 0.013) than MDD − A. Other dimensions had no significant differences between two groups (all p > 0.05) (Figure 3B).
3.2.3 Interoception predict depressive symptoms
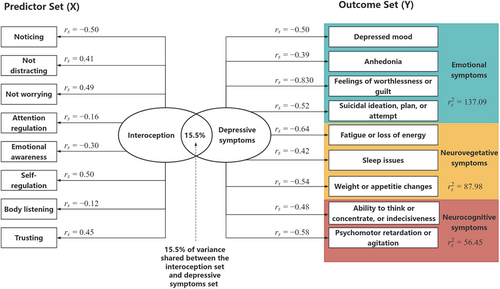
The CCA was conducted to evaluate the multivariate shared relationship between the interoception predictors set and depressive symptoms outcomes set. The correlation coefficients of the first pair of canonical variates were statistically significant (p < 0.05), with the first correlation coefficient having a value of 0.394, an eigenvalue of 0.184, a contribution rate of 60.53% (F = 1.91, p < 0.001). The effect size (1 − Λ) for the full model was 0.155, suggesting that a substantial 15.5% of variance was shared between interoception dimensions and depressive symptoms.
Subsequently, the structure coefficients (rs) and squared structure coefficients () of individual variables were evaluated to determine which variables contributed to the relationships between sets of variables. of emotional symptoms, neurovegetative symptoms, and neurocognitive symptoms were 137.09, 87.98, and 56.45.3
“Self-regulation”, “noticing” and “not worrying” were the primary drivers among interoception dimensions, whereas PHQ6 from emotional symptoms ( = 69.22), PHQ4 from neurovegetative symptoms ( = 41.47), and PHQ8 from neurocognitive symptoms ( = 33.41) were the primary drivers among the depressive symptoms (Table 2 and Figure 4).
| Variables | Full model | Symptoms () | ||
|---|---|---|---|---|
| Coef | r s | (%) | ||
| Outcomes (depressive symptoms) | ||||
| PHQ1: Depressed mood | −0.10 | −0.50 | 25.00 | Emotional symptoms (137.09) |
| PHQ2: Anhedonia | 0.24 | −0.39 | 15.52 | |
| PHQ6: Feelings of worthlessness or guilt | −0.60 | −0.83 | 69.22 | |
| PHQ9: Suicidal ideation, plan, or attempt | −0.09 | −0.52 | 27.35 | |
| PHQ3: Sleep issue | −0.12 | −0.42 | 17.89 | Neurovegetative symptoms (87.98) |
| PHQ4: Fatigue or loss of energy | −0.30 | −0.64 | 41.47 | |
| PHQ5: Weight or appetite changes | −0.20 | −0.54 | 28.62 | |
| PHQ7: Ability to think or concentrate, or indecisiveness | −0.05 | −0.48 | 23.04 | Neurocognitive symptoms (56.45) |
| PHQ8: Psychomotor retardation or agitation | −0.23 | −0.58 | 33.41 | |
| Predictors (interoception dimensions) | ||||
| Noticing | −0.26 | −0.50 | 24.50 | |
| Not distracting | 0.35 | 0.41 | 17.06 | |
| Not worrying | 0.19 | 0.49 | 24.11 | |
| Attention regulation | −0.23 | −0.16 | 2.56 | |
| Emotional awareness | −0.22 | −0.30 | 8.76 | |
| Self-regulation | 0.67 | 0.50 | 25.10 | |
| Body listening | −0.19 | −0.12 | 1.49 | |
| Trusting | 0.40 | 0.45 | 19.98 | |
- Note: The categorization of the depressive symptoms include Emotional symptoms, Neurovegetative symptoms, and Neurocognitive symptoms. Coef, standardized canonical function coefficient; rs, structure coefficient; , squared structure coefficient—percent of variance shared between the individual variable and the variable set.
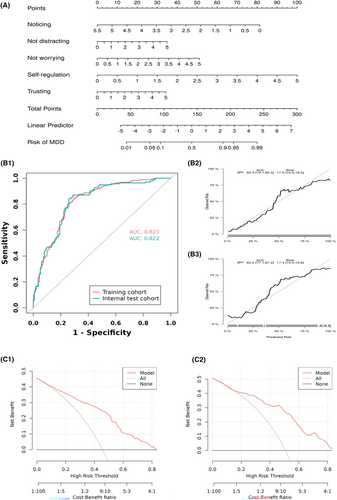
3.2.4 Diagnostic efficacy of interoception for MDD
Five potential predictors (noticing, not distracting, not worrying, self-regulation, body listening, and trusting) were selected by LASSO regression analysis (Appendix S2). The final logistic model is developed as a simple-to-use nomogram, which is illustrated in Figure 5A. ROC analysis revealed that AUC value of the nomogram reached 0.823 (CI: 0.795–0.858) in training cohort and 0.822 (CI: 0.796–0.890) in internal test cohort, indicating that this model has stable and good discriminant ability (Figure 5B1). The calibration plots of the nomogram in the different cohorts demonstrated a good correlation between the observed and predicted MDD status (Figure 5B2,B3). DCA curves indicated that using this model is better than no intervention at all for all threshold levels in both cohorts, showing that the nomogram is effective in clinical practice (Figure 5C).
4 DISCUSSION
This is the largest study to date investigating interoception deficits at different stages of MDD and their correlation with depressive symptoms. Previous research on interoception in MDD was constrained, particularly in understanding interoception dysfunction during various MDD stages. Furthermore, the multidimensional nature and individual variability of interoception also complicated the research.46-48 Our study addresses these gaps by focusing subclinical and drug-naïve MDD population and collecting more comprehensive demographic data. We found that individuals demonstrated interoceptive deficits prior to realizing their depressive state (in SD stage), characterized by heightened concern for unpleasant sensations and a reduced capacity to distract attention from discomfort. In the clinical stage of MDD, the range of interoception impairment dimensions was more severe. Specifically, MDDs were hypersensitive to body sensations (higher “noticing”), unable to focus on and regulate physical sensations (lower “attention regulation” and “self-regulation”) and had difficulty in interpreting and trusting bodily cues (“lower body listening” and “trusting”). The interoception dysfunction in various stages made it an early diagnostic and intervention marker for MDD. Additionally, it could also explain the observed efficacy of mindfulness-based cognitive therapy (MBCT), which is rooted in interoceptive principles, in treating SD and MDD, thereby substantiating the role of interoception in MDD management.49-52 However, the interoception dysfunction identified in MDDs partially diverged from prior research. While reductions in “body listening” and “trusting” were consistent with earlier study, we observed an increased notice to bodily signals in MDDs, which contrary to the findings of Dunne et al.53 Existing literature highlighted that MDDs exhibited heightened pain sensitivity,54, 55 and were prone to comorbid interoception dysfunction conditions like irritable bowel syndrome.56, 57 Animal experiments also showed the enhanced pain sensitivity in mice under long-term chronic stress.58, 59 Consequently, we concluded that MDDs should have a heightened capacity for bodily information perception. It is noteworthy that Dunne's research focused on depressive symptoms in primary care patients, limiting its applicability across different populations and healthcare setting.53 Interestingly, our study revealed that while MDDs exhibited slight higher levels of “emotion awareness” compared with HCs, the difference was not statistically significant and appeared unrelated to medication. This indicated that MDDs had stronger emotional reactions to physical discomfort (significance in “no worrying”), but their perception and interpretation of somatic information induced by the purely emotional experience might be similar to HCs (no significance in “emotion awareness”). Bodily experiences could play a more significant role in contributing to MDDs' emotional problems than we previously thought.60 Residual somatic symptoms have always been a difficulty in antidepressant treatment and an important factor in the recurrence of depression.52, 53, 61, 62 Our findings suggest that strategies relying solely on emotional therapy to alleviate somatic symptoms might have limited efficacy. Further research is required to comprehensively understand the connection between mind and body.62
In the investigation of antidepressant treatment effects on interoception, results showed that MDD with antidepressive therapy were more likely to disregard uncomfortable bodily sensations compared with without antidepressive therapy. However, their ability to listen to the body for cues or insight and calming one's own down by paying attention to bodily information were further impaired. These results suggested that MDDs with antidepressive therapy exhibited a blunted interoceptive pattern. While this blunted interoception might alleviate excessive focus on uncomfortable bodily sensations, it seemed to exacerbate the impairment of other interoceptive regulation capabilities. Lyons' research yielded similar conclusions,31 indicating that treated MDDs have weaker somatic responses in emotional contexts. Antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), are known to inhibit exteroceptive processing,63, 64 alleviate visceral pain,65 increase emotional “blunting”,66 reduce responses to reward and punishment,67 and increase amygdala and insular responses to threat cues.68 These evidences highlight the inhibitory effect of antidepressants on the general neural response to interoception.69 Additionally, it suggests that current antidepressive therapy may achieve their effects by damaging interoceptive functions. Therefore, investigating the interoceptive functions in MDD is essential. Integrating new interoceptive-based therapeutic interventions could be a potentially effective strategy for MDD treatment and prevention.
Currently, intervention strategies targeting interoception have shown promising results. MBCT effectively enhances emotional regulation and psychological resilience by increasing interoceptive awareness.70, 71 However, its efficacy may be limited in MDDs during acute episodes due to impaired meditation ability.72, 73 Conversely, body psychotherapy and massage therapy, which enhance the mind–body connection through physical interventions,74-77 have demonstrated significant efficacy in managing MDD. It indicates that bodily engagement in the treatment can directly influence mental health. Based on this, Lyons et al.60 introduced an innovative interview technique aimed at exploring bodily experiences related to MDD. This technique focuses on promoting deep introspection of somatic sensations in MDDs, providing further insights into how bodily experiences shape depressive episodes. These findings underscored the clinical utility of interoception research in depression.
We further identified a multivariate correlation between interoception and depressive symptoms. Contrary to our hypothesis that interoception impacted depression through somatic symptoms, interoceptive dimensions were found to affect the breadth of depressive symptoms, illustrating the potential of interoception in explaining MDD's symptomatic heterogeneity. In the current research, interoception primarily accounted for emotional symptoms, particularly feelings of worthlessness or guilt.17, 78 This indicated that interoception mainly lead to MDD by enhancing negative thinking patterns,47 aligning with our prior neurophysiological study's findings, i.e., the altered cardiac interoception contributed to the aberrant encoding of negative emotional faces in MDD, mediated by atypical activation in the right insula and anterior cingulate cortex (ACC).79 This clinical study reinforced these experimental results that indicated interoception is involved in the pathogenesis of MDD.
Ultimately, our study evaluated the role of interoception in identifying MDD with an internal validation. Key interoceptive factors—self-regulation, noticing, not worrying, trust, and not distracting—reliably separated MDDs from HCs in our datasets. These dimensions were also identified as the major five interoception predictors of depression within CCA. Additionally, we used DCA to supplementarily assess the utility of the model in actual clinical decision-making. The results reaffirmed the role of interoception in improving the clinical net benefit for MDDs.
In summary, we effectively tested the ability of interoception to change clinical practice in MDD according to a standardized process.34 Our findings highlighted the importance of considering interoception as integral to the diagnosis and treatment of MDD. Future research on interoception's brain mechanisms in MDD and the creation of targeted clinical interventions are vital for better diagnosis and management of MDD. Importantly, from the perspective of neuroimaging and neuroelectrophysiology, studies are needed to further clarify the role of interoception in the pathogenesis of MDD.
The study had three principal limitations. First, its observational nature limited understanding of interoception's role in MDD, indicating the need for future large-scale, longitudinal, and multi-center studies by a prospective cohort design. Second, the present research did not extend to interventional studies on interoception in depression. Supplementing with intervention research will help us further clarify the role of interoception in MDD. Given the potential impairment of interoception, future research should focus on the effects of different antidepressants on interoception. Finally, the impact of demographic differences, such as gender and cultural variations, requires detailed investigation in the future. Examining specific bodily experiences (e.g., head, stomach, heart) could also provide deeper insights into the role of bodily information in the pathophysiology of depression.
5 CONCLUSIONS
Our findings demonstrated a potential progressive exacerbation of interoception impairments throughout MDD progression, suggesting their potential as early markers for diagnosis and intervention. Given the modest impact of traditional antidepressive therapies on these impairments, our findings highlighted the need for integrating interoception into treatment strategies. Our study also identified a complex multivariate correlation between interoception and depressive symptoms, advancing our understanding of the complex physiological–psychological interplay in MDD pathogenesis which shed light on depression's heterogeneity. Importantly, internal validation confirmed the strong discrimination and predictive power of interoception, reinforcing its value in refining diagnostic accuracy and personalizing treatment. Overall, our findings highlighted the importance of interoception in MDD management, pointing towards a paradigm shift in diagnostic and therapeutic approaches.
AUTHOR CONTRIBUTIONS
We extend our sincere gratitude to Zhenhe Zhou for his financial support. Hongliang Zhou played a pivotal role in conceptualizing the study, analyzing data, and drafting the manuscript. Chenguang Jiang contributed to the manuscript writing. Jikang Liu and Yuqing Wu were responsible for recruiting depression subjects and conducting psychiatric assessments. Zixuan Huang, Wenliang Wang, Yuhang Ma undertook the community recruitment.
ACKNOWLEDGMENTS
We wish to express special gratitude to the individual participants in this study.
FUNDING INFORMATION
This study was supported in part by grant no. 202107 from Wuxi Municipal Health Commission Major Project. The funder approved the study protocol prior to study initiation. The funder had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data will be available to researchers with a signed data access agreement. Data will be made available to researchers whose proposed use of the data has been approved. Data will be made available for any purpose. Data will be available to researchers by [email protected].




